107b Cell-Matrix Interactions: Quantifying Cell Migratory and Contractile Behavior in a Series of Characterized Collagen-Gag Scaffolds
Quantitative study of individual cell behavior within a three-dimensional construct requires understanding the local environment of individual cells through accurate characterization of the scaffold chemical composition, porous microstructure, and mechanical properties. The objective of this study was to use two series of collagen-GAG (CG) scaffolds independently varying mean pore size and stiffness [1], to study their independent effect on cell migration speed and individual cell contractile force.
CG scaffolds were fabricated via lyophilization from a slurry of type I collagen and chondroitin 6-sulfate in acetic acid [2,3]. A series of uniform scaffolds with homogeneous pore structure and equiaxed pores, constant composition and relative density (0.6%), but with distinct pore sizes (151, 121, 110, 96 Ám) have been produced [2,3]. All scaffolds were crosslinked via dehydrothermal (DHT) crosslinking (105oC, 24 hours, <50 mTorr) [2,3]. Mechanical characterization demonstrated scaffold isotropy; the hydrated scaffolds have a compressive modulus of 208▒41Pa independent of pore size. Scaffold stiffness can be modified independent of pore size by modulating crosslinking density. Two carbodiimide (EDAC) crosslinking intensities were utilized to increase scaffold stiffness relative to DHT crosslinking [4]: EDAC 1:1:5 (2.0x stiffer than DHT) and EDAC 5:2:1 (7.2x stiffer).
NR6 mouse fibroblasts and DU-145 prostate cancer cells were fluorescently labeled with 10 ÁM CMFDA Cell Tracker (Molecular Probes); CG scaffolds were fluorescently labeled with 2 ÁM Alexa Fluor 633 (Molecular Probes). Cells (NR6 or DU-145) were then seeded into 6 mm (dia.) scaffold disks and imaged using the Perkin Elmer Ultraview Live Cell Imager at 15 minute intervals for 10 hours using a heated (37oC) 25x oil-immersion objective. A 3D image rendering software package (Imaris XT, Bitplane AG) was used to determine cell centroid displacement over time to calculate cell migration speed. Combining strut mechanical characterization data with the observed strut deformation during contraction allowed calculation of contractile forces generated by individual cells within the scaffold.
A robust system has been developed allowing analysis of cell behavior within well-characterized scaffolds that allow independent manipulation of microstructure and mechanics. Individual fibroblasts were calculated to generate contractile forces of 11-34 nN. NR6 and DU-145 cells were observed to migrate through the scaffold with an average speed between 4 and 13 Ám/hour; a significant effect on cell migration speed of scaffold mean pore size, stiffness, cell seeding density, and cell type was observed. Migration speeds were of a similar magnitude as those reported for studies of cell motility on 2D membranes [5]. Measured cell-mediated contractile forces were significantly larger than those previously reported in CG scaffolds, likely because previous studies reported average values calculated from cell populations in which not all cells were actively contracting rather than values from individual cells.
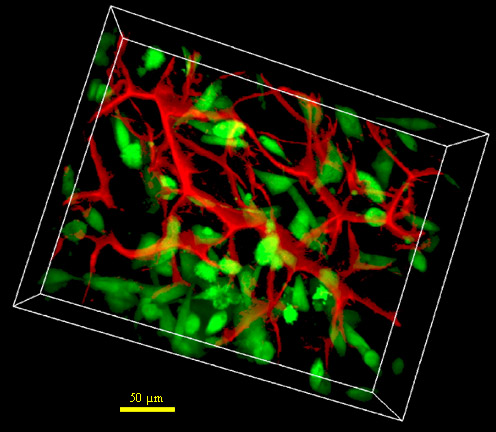
Fig. 1. 3D imaging of NR6 fibroblasts (green) within the CG scaffold (red). Scale bar: 75 Ám
References:
[1] B.A. Harley, F.J. O'Brien, I.V. Yannas, and L.J. Gibson, Trans. Soc. Biomater. 30, 2004.
[2] F.J. O'Brien, B.A. Harley, I.V. Yannas, L.J. Gibson, Biomaterials, 26, 2005.
[3] O'Brien FJ, Harley BA, Yannas IV, Gibson LJ. Biomaterials 25, 2004.
[4] Lee CR, Grodzinsky AJ, Spector M. Biomaterials 22, 2002.
[5] Lo CM, Wang HB, Dembo M, Wang YL. Biophys J, 79, 2000.
Keywords: scaffolds, cell behavior, migration speed, contraction