282g Engineering Outer Membrane Vesicles for Gene Delivery
A key issue in non-viral gene therapy is the design of vectors that can effectively overcome the cellular barriers to nucleic acid delivery. Particle sizing and surface charge analyses of OMVs show native vesicles to have a hydrodynamic diameter of approximately 100 nm, and a zeta potential of -12 mV. Although the vesicle size is ideally suited for endocytosis by cells, the nonspecific uptake of native OMVs is currently precluded by the net negative surface charge. Consistent with established design parameters for synthetic polycation delivery vectors, we have focused on engineering OMVs with a net positive surface charge to enhance mammalian cell uptake.
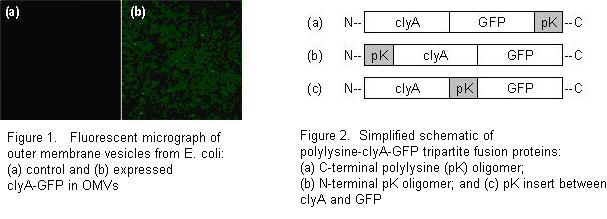
Towards the goal of an OMV-based DNA delivery vector, we have modified the surface properties and lumenal contents of OMVs by recombinant DNA technology. OMVs were first labeled with green fluorescent protein (Figure 1) by fusion of GFP with the clyA protein. ClyA is found to be preferentially enriched in outer membrane vesicles. In an effort to overcome the existing net negative surface charge of OMVs, we have created a set of polylysine-clyA-GFP tripartite fusion proteins to be expressed on the vesicle surface. Since the spatial orientation of this protein within the vesicle membrane is currently unclear, three variants of the polylysine fusion proteins were created: an N-terminal polylysine addition, a C-terminal polylysine addition, and a polylysine linker connecting the clyA and GFP regions (Figure 2). The expression level of these fusion proteins may be conveniently detected by direct green fluorescence. Our results indicate that the expression of the polylysine-clyA-GFP fusion proteins on the vesicle surface may lead to modulation in the zeta potential of OMVs. These fluorescent engineered OMVs enable visualization in future in vitro cellular trafficking studies of vesicles in association with mammalian cells. This initial work illustrates that surface modification of OMVs can alter their interactive potential with mammalian cells, offering possibilities of OMVs designed with a variety of surface-expressed moieties for vaccines and therapeutics.
[1] Kesty, N.C., Mason, K.M., Reedy, M., Miller, S.E., and Kuehn, M.J. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23: 4538-4549 (2004).