559a Reaction Mechanisms and Kinetic Modeling of Ternary Thiol-Vinyl Photopolymerizations
Photopolymerization is one of the most rapidly expanding processes for materials production, with more than 20% annual growth in a multibillion dollar industry that is employed over a wide range of fields. It is one of the most energy efficient processes known and can be used as a 100% solvent free process for extremely rapid, pollution-free curing of polymer coatings from liquid monomers. However, the applicability and utility of the traditional acrylic and methacrylic systems is currently limited due to several disadvantages associated with the polymerization. Thiol-vinyl photopolymerizations, which are free radical polymerization reactions between thiol (-SH) containing monomers and vinyl containing monomer (-C=C), represents a radical shift in polymerization mechanism that addresses all the disadvantages associated with acrylic polymerizations. The binary thiol-vinyl polymerization scheme leads to significant advantages related to reduction/elimination of oxygen inhibition, delayed gelation, lowered volume shrinkage and shrinkage stress, homogeneous network evolution, and high functional group conversions. Employing ternary thiol-vinyl systems (thiol-ene-ene and thiol-ene-acrylate systems) further offer several distinct additional advantages for thiol-vinyl photopolymerizations. These advantages are primarily due to enhanced control and tailorability over polymerization kinetics and polymer material properties. These ternary thiol-vinyl formulations also provide a unique framework through which control over curing kinetics transforms into control over polymer network architecture. To control the relative consumption of individual components in ternary systems and thereby impact network properties, it is essential to be able to predict the polymerization kinetics in these systems. In this work, we utilize rate parameters from binary thiol-ene systems for predicting the ternary polymerization kinetics.
The reaction kinetic parameters in binary thiol-vinyl systems were first quantified by utilizing modified unsteady state rotating sector experimental technique. In order to utilize this technique to quantify kinetic parameters, analytical equations were developed to express thiol-vinyl unsteady state polymerization rates. Kinetic parameters in several binary thiol-vinyl systems were determined by utilizing the analytical expressions for steady state and unsteady state polymerizations and experimental knowledge of ts from rotating sector analysis. Although the polymerization kinetics of thiol-vinyl systems has been long investigated, our current work is the first study to have the ability to quantify the kinetic parameters in these systems and to demonstrate the relative importance of termination events.
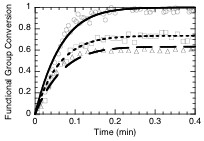
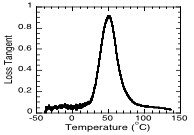
The kinetic parameters determined through unsteady state analysis of binary systems were employed for the prediction of curing kinetics of ternary thiol-vinyl systems. Utilizing a polymerization kinetics model developed for ternary thiol-vinyl systems and the reaction kinetic parameters for the corresponding binary systems, the polymerization kinetics of several thiol-ene-ene and thiol-ene-acrylate systems were successfully predicted. For example, Figure 1 presents the experimental polymerization kinetics of one particular ternary thiol-vinyl system, thiol-vinyl ether-norbornene system, along with modelling predictions. Ternary thiol-vinyl systems were shown to provide several additional advantages over traditional binary thiol-vinyl systems. These advantages include, enhanced consistency of material properties with small changes in monomer compositions, superior mechanical properties (Figure 2), and unique opportunities for photolithographic development of holographic patterns.
An ability to predict the relative consumptions of monomers in ternary systems would facilitate understanding of network evolution in these systems. Further, it would lead to improved monomer chemistry variation, in which we are able to control the network properties of the formed networks.
|
|
|
| Figure 1: Model predictions and experimental data for conversion versus time of ternary thiol-vinyl-norbornene photopolymerizations. Thiol (O,—), vinyl ether (r,– –),and norbornene (,---) conversions for initially 1:1:0.5 stoichiometric ratios of thiol: vinyl: norbornene functionalities. All the kinetic parameters employed for modeling are experimentally determined | Figure 2: Loss tangent curve of a ternary thiol-vinyl systems, demonstrating its superior material property of high glass transition temperature (Tg) as well as narrow Tg width. The ternary system is formed from 1:1:2 stoichiometric ratios of tetrathiol, divinyl ether, and Tricyclodecanediol diacrylate monomers. |