171f Development of Microvascularized Tissue-Engineered Products: Flow Characterization and Scaffold Fabrication
Choosing skin as the tissue model and utilizing planar bifurcating networks as our basic design, we previously obtained optimal designs of micron scale flow networks. In this work, we will present results of network flow characterization studies, including flow distribution and pressure drop measurements across the network devices. We will also present preliminary results of network fabrication in collagen-glycosaminoglycans (collagen-GAG) scaffolds and network endothelialization.
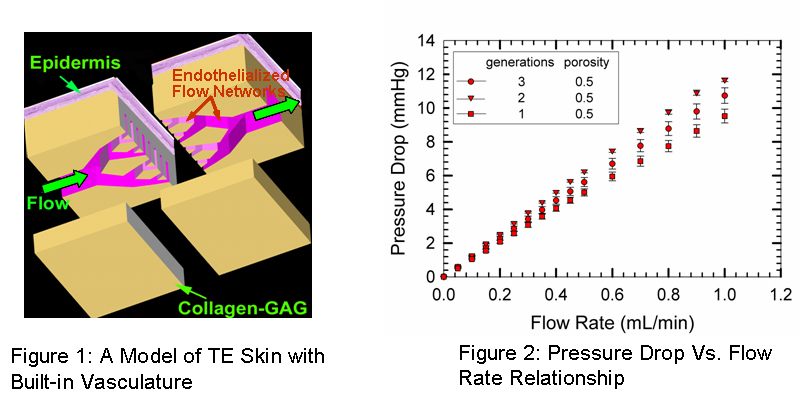
By using standard photo/soft lithography techniques, the network designs were obtained in poly-dimethyl siloxane (PDMS) molds. Subsequently, microfluidics was established in the PDMS networks and the differential pressure drop across the network was measured using an amplified low pressure sensor. The pressure drop-flow rate relationship was obtained as a function of network generations and network porosity. Figure 2 shows preliminary results for 1-3 generation devices. All devices obey the Poiseuille flow behavior. In addition, flow velocities were measured at different segments of the network devices using particle image velocimetry and the flow distribution efficacy of the networks was quantified. We developed a new soft-lithography technique for fabricating the micron scale flow networks onto collagen-GAG scaffolds. Four process parameters were optimized to obtain well-resolved and stable features: acetic acid (AA) solution concentration, surface dissolution time, applied pressure and glutaraldehyde concentration. The collagen-GAG networks were subsequently endothelialized with BAEC's and cell growth and viability were assessed. Collectively, the results validate our approach for incorporating synthetic ‘vascular' networks with TE products.