476ao Promoting the Preferential Oxidation of Co by Altering the Reducibility of Pt
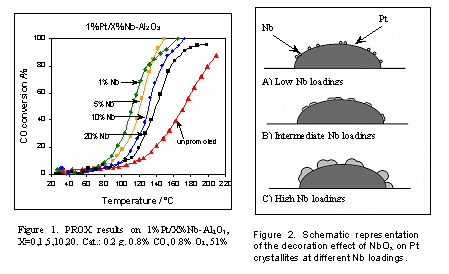
The promotion of Pt/Al2O3 with niobia (referred hereafter as Nb2O5 unless otherwise indicated) has been studied during the preferential oxidation of CO in the presence hydrogen (PROX reaction). The catalysts were prepared by co-precipitacion of the metals precursors (tetraammineplatinum(II) nitrate and ammonium niobium oxalate) on a solution of finely dispersed alumina. CO conversion vs temperature on 1%Pt/x%Nb-Al2O3 (x=0,1,5,10,20) catalysts, show that at lower Nb loading the activity increases. When yields were compared, a maximum of 65% CO2 produced was obtained for the 5%Nb-promoted catalyst. Operando infrared results show a shift to higher frequencies in the linear CO band as Nb loading is increased. FTIR shows that under reaction conditions this band has a spectator character and does not change as the extent of the reaction increases. Operando EXAFS/XANES and ex-situ XPS results show that the reduced Pt Nb-promoted catalysts exhibit Pt+2 species. In fact, in all the Nb-promoted catalysts, 40% of Pt remains as Pt+2 when pre-reduced in hydrogen at 250şC, while unpromoted Pt/Al2O3 was completely reduced at the same temperature.Interestingly, the existence of oxidized Pt species does not affect the catalytic performance. XPS also shows lower oxidation states for surface NbOx when compared with bulk Nb2O5. At low loading, niobia species are anchored to the support by titrating surface hydroxyls which form four-fold coordinated surface NbO4 [1-5]. These highly dispersed NbOx species could explain the lower oxidation state for Nb when supported on Al2O3. Niobia is also known for gradually titrating the surface hydroxyls from the Al2O3 support, and completely eliminating them above monolayer coverage [6,7]. Under these conditions on the Pt-promoted catalysts, niobia may decorate the Pt surface, altering its chemistry. For catalysts with similar Pt crystallite size, H2 chemisorption shows that the amount of H2 adsorbed decreases as Nb loading is increased. On the basis of the operando results we hypothesize that the presence of small-unsaturated NbOx species may decorate the Pt surface at different Nb loadings (Fig. 2). As Nb loading increases the decoration effect also increases and bigger aggregates of NbOx are formed. The same Pt particle size gives lower H2 adsorption when Nb loading increases. At higher Nb loadings, these aggregates of NbOx could be hindering H2 adsorption on Pt. It also should be noticed that for finely dispersed NbOx, the interface between NbOx and Pt increases at low Nb loadings. This region of strong interaction between niobia and Pt could be playing a role in the activity-selectivity of the catalysts, since niobia is known for having a redox behavior [8]. This reduction-oxidation cycle on niobia, interacting with the neighboring adsorbed CO adsorbed on Pt might explain the promoter effect of low loading of Nb for the preferential oxidation of CO. When 1%Pt was supported on Nb2O5 the CO conversion was very low in the preferential oxidation reaction, but when only CO and O2 were reacting, the typical CO ignition behavior on platinum catalysts was observed. In contrast, when H2 was absent the CO oxidation activity was restored. This clearly shows an inhibiting effect of H2 on CO oxidation on 1%Pt/Nb2O5. In summary, this work shows that by adding niobia to Pt/Al2O3 catalysts, the catalytic activity on the preferential oxidation of CO can be improved. The semi-oxidized catalysts promoted with niobia show higher activity for the preferential oxidation of CO than the fully reduced unpromoted Pt/A2O3 catalyst. [1] Israel E. Wachs, Catalysis Today, 27 (1996): p. 437-455. [2] S.M. Maurer, D. NG, and E.I. Ko, Catalysis Today, 16 (1993): p. 319-331. [3] P.A. Burke, and E.I. Ko, Journal of Catalysis, 129 (1991): p. 38-46. [4] S.M. Maurer, and E.I. Ko, Catalysis Letters, 12 (1992): p. 231. [5] X. Gao, I.E. Wachs, M.S. Wong, and J.Y. Ying, J. Catal. 203 (2001): p. 18-24. [6] A. M. Turek, and I.E. Wachs, J. Phys. Chem. 96 (1992): p. 5000-5007. [7] J. M. Jehng, and I. E. Wachs, J. Molec. Catal. 67 (1991): p. 369-387. [8] I.E. Wachs, J.M. Jehng, G. Deo, H. Hu, and N. Arora, Cat.Today 28(1996): p. 199.