600c Polymeric Microneedles Encapsulating Drug for Controlled Release Delivery
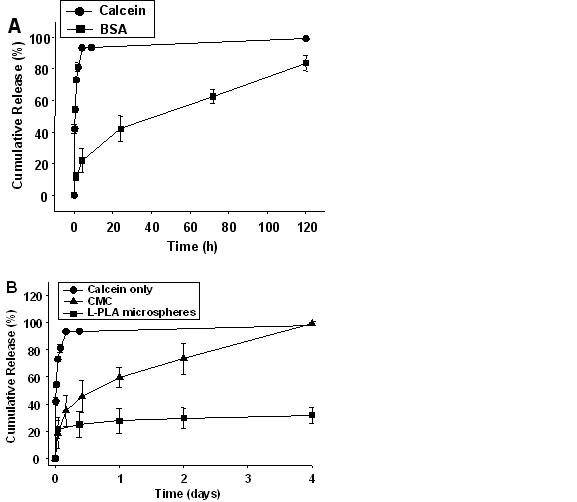
A limitation of present controlled release systems is that they typically require hypodermic needle injection of polymeric microparticles or possibly surgical implantation of macroscopic devices within the body. These painful and invasive procedures are generally not suitable for self-administration by patients and therefore are limited to use in hospitals or clinics. The goal of this study was to develop a minimally invasive polymeric controlled release system suitable for self-administration without the pain or complexity of current controlled release devices. Rather than using a hypodermic needle to introduce polymeric microparticles into the body, we propose redesigning the microparticles to have the shape of microneedles and thereby give these polymeric particles the functionality of both needles and drug matrices for controlled release. Drug was encapsulated in polymer microneedles that are designed for controlled release in skin and that are suitable for self-administration by patients as an alternative to hypodermic injection or implantation of controlled release systems. Arrays of microneedles were fabricated out of poly-lactide-co-glycolide (PLGA) using a mold-based technique to encapsulate model drugs – calcein and bovine serum albumin (BSA) – either as a single encapsulation within the needle matrix or as a double encapsulation, by first encapsulating drug within carboxymethylcellulose or poly-L-lactide microparticles and then encapsulating drug-loaded microparticles within needles. PLGA microneedles exhibited sufficient mechanical strength to insert into human skin and were able to encapsulate drug at a mass fraction up to 10%. In vitro release of encapsulated drug was controlled by the encapsulation method and formulated to achieve release kinetics ranging from hours to months. After microneedle fabrication at elevated temperature, up to 90% of encapsulated BSA remained in its native state. We conclude that biodegradable polymer microneedles can encapsulate drug to provide controlled release delivery in skin.