547b Catalytic Activities of Mixed Conductive Bi2ti0.1v0.9o5.45 for H2/Methanol Oxidation in Acid Aqueous Solution
The mixed conductive Bi2V0.9TiO0.1O5.45 was found to have similar catalytic ability with a porous Pt catalyst for methanol oxidation and for oxygen reduction above temperatures of 350oC [1]. If the catalytic ability of Bi4Me0.1V1.9O10.95 at temperature below 100oC is also similar or even slightly low than Pt, Therefore, it is possible to replace Pt by the cheap ceramics as the catalyst in the fuel cell, which will greatly decrease the cost of the fuel cells. However, the main challenge to the BiMeVOx as the catalyst in the fuel cell is the poor electronic conductivity. In this paper, Bi2V0.9TiO0.1O5.45 (abbreviating to BiTiVOx as below) powder with high electronic conductivity was synthesized and its catalytic activities to H2/methanol oxidation in acid aqueous solution investigated.
Using Bi203, V205 and TiO2 as the starting materials, the BiTiVOx was prepared by solid state reaction [2]. Differing with the conventional synthetical route, the material was heated at 800oC and modified it into mixed conductor. The heat treated BiTiVOx shows both a high electronic conductivity (0.005 S/cm) and an ionic conductivity (0.002 S/cm) at room temperature, which was determined using electrochemical impedance spectroscopy (EIS). To evaluate the catalytic activities of the BiVTiO, the porous electrode was fabricated by painting the inks mixed with the BiTiVOx with 5% Nafion solution in the mass ratio of 10:1 onto the surface of carbon cloth. For comparison, the Pt electrode was fabricated with the same way by using 40% Pt/C catalyst and the mass ratio of the Pt/C to Nafion 3:1.
Fig.1 shows the polarization characterization of the BiTiVO and Pt/C in the 1M H2SO4 solution with H2 bubbling (simulating the anodic circumstance in the PEMFC) and in the 1M H2SO4 +1M CH3OH solution (simulating the anodic circumstance in the DMFC), respectively. In 1M H2SO4 solution with H2 bubbling, the catalytic activity of the BiTiVOx to H2 reduction increases with the active material loading from 7.6mg/cm2 to 105mg/cm2, and the latter is almost the same with that of the Pt/C catalyst loaded 0.6mg/cm2. The results indicate that the BiTiVOx with high electronic conductivity has a good catalytic ability to reduce H2 in the acid solution. In 1M H2SO4+1M CH3OH solution, the catalytic ability of the BiTiVOx for methanol reduction is much higher than the Pt/C catalyst. Pt catalyst catalyze the methanol oxidation only at overpotential higher than 0.3 V, while methanol can be oxidized on the BiTiVOx catalyst at a very low overpotential and the produced current on BiTiVOx catalyst at a low potential (0-0.3 V) is much higher than that of Pt catalyst. Moreover, the low potential of the BiTiVOx for methanol reduction will lead to a high working voltage of the DMFC and thus increases the efficiency of the fuel cell.
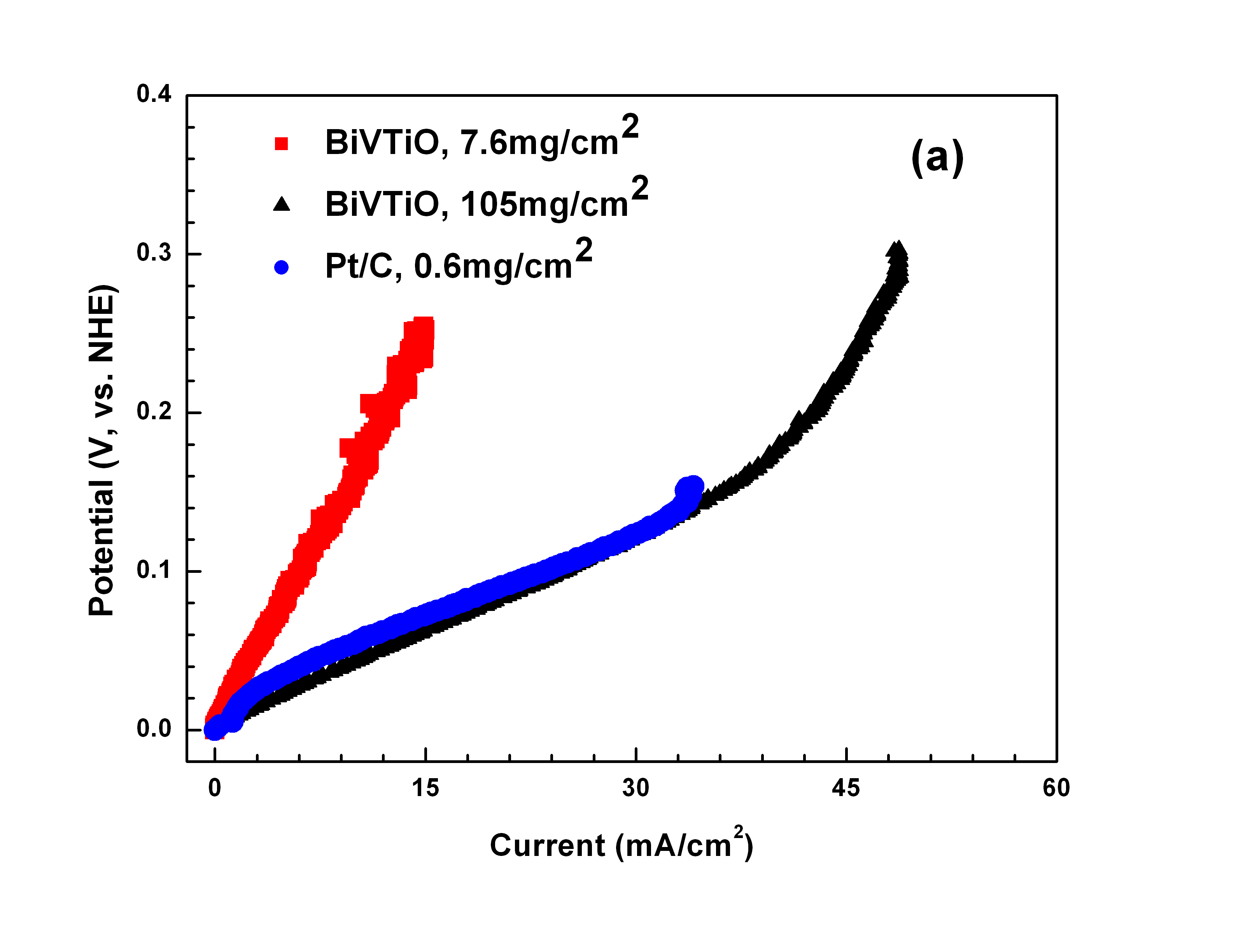
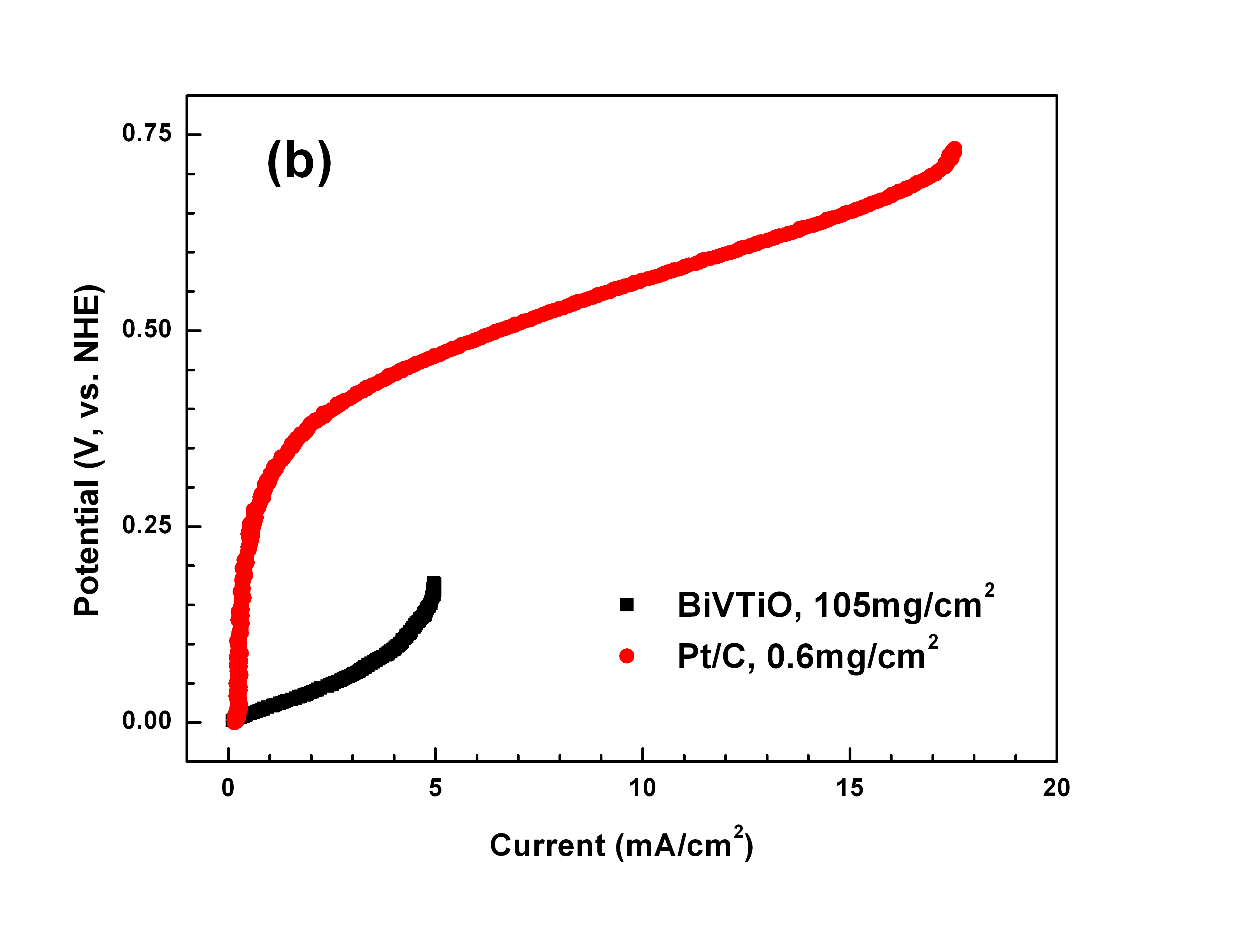
Fig1. Comparison of the polarization performance of the Bi2V0.9TiO0.1O5.45 catalyst with that of Pt/C catalyst tested in (a) 1M H2SO4 solution with H2 bubbling, and (b) 1M H2SO4+1M CH3OH solution
References
1. B. A.Boukamp, Solid State Ionics, 136, 75(2000)
2. I. Abrahams, F. Krok, M. Malys, A.J. Bush, J. Mater. Sci. 36 (2001) 1099