336b A Novel, Quantifiable Three-Dimensional Model of Melanoma Invasion
The majority of observations regarding melanoma growth have been made in two-dimensional (2D) in vitro systems or in situ in human patients or animal models. A true three-dimensional (3D) in vitro system that combines the advantages of 2D and in situ models (controllable environment and appropriate mechanical cues, respectively) is lacking.
To address this need, our group has developed a 3D fibrin-based assay to investigate the invasive characteristics of three disparate stages of melanoma. Using image processing software, we have also developed a method to track and quantitatively assess the extent to which single populations of these cells invade their surrounding matrix.
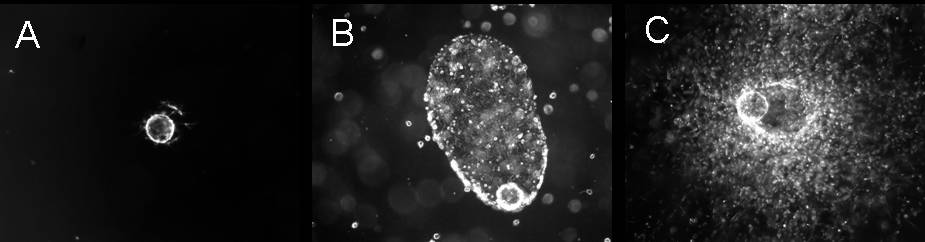
Human epithelial melanocytes of neonatal origin (HEMn), an amelanotic melanoma cell line (M14), or a highly metastatic melanoma cell line (C8161.9) were coated on microcarrier beads and gelled within fibrin matrices of different compositions. Images of isolated microcarrier beads obtained at days 1, 2, 3, and 7 (day 7 images for A) HEMn, B) M14, and C) C8161.9 shown in figure demonstrate distinct patterns of invasion) were sharpened, thresholded, and traced to determine a percent invasion into the surrounding field. Varying the fibrin concentration from 3 mg/ml (the approximate circulating concentration of fibrinogen) to 12 mg/ml (approaching the reported ECM density surrounding a tumor) demonstrated significantly decreased invasion of C8161s (as determined by percent invasion). However, addition of collagen to the dense matrix condition restored the invasive capacity of this cell line. Invasive characteristics were also monitored in the presence and absence of dermal fibroblasts (DF) in these matrix conditions. Removal of DF led to widespread, uncoordinated proliferation of both melanoma cell lines, while HEMn were largely unaffected. Lastly, the use of this system as a quantitative assay for drug screening was demonstrated by applying doses of known protease inhibitors to gauge the proteolytic dependence of each cell type. C8161s were more dependent on MMPs while M14s and HEMn relied primarily on plasmin to invade fibrin matrices.
These data serve as the initial steps in characterizing a novel 3D system to comparatively study melanoma invasion and demonstrate its potential use to quantitatively gauge the effects of pharmacological agents on melanoma growth and invasion.