211d New Silicoaluminophosphate and Titanosilicate Nanoporous Sorbents for Gas Phase Separations
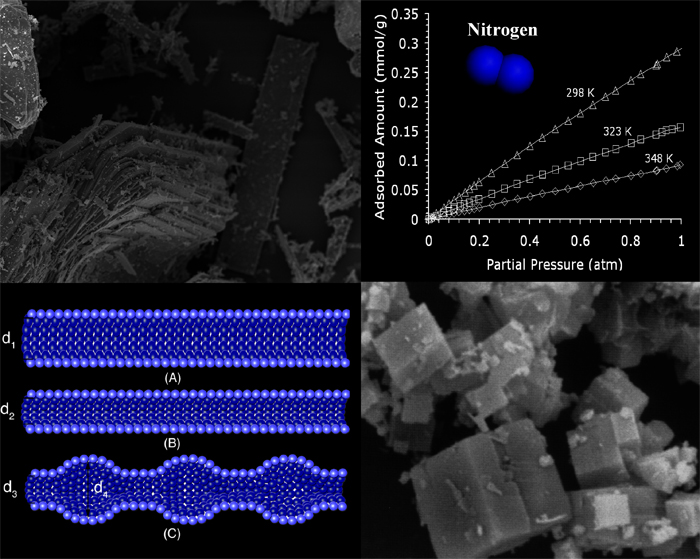
Modified and unmodified SAPO-34 nanoporous materials have been tested for adsorption of nitrogen (N2) and methane (CH4), respectively, via equilibrium and diffusion kinetic methods. Our data indicate that the isosteric heat of adsorption is approximately -22.03 kJ/mol for N2 and -12.18 kJ/mol for CH4, which correlates to physical and reversible adsorption. It should be mentioned that a Langmuir isotherm model predicts (by extrapolation) a 3.64 wt% N2 adsorption capacity at 298 K and higher pressure range (~10 atm). The predicted saturation capacity is larger than the one offered by many commercially available sorbent materials. Ion exchange in SAPO-34 with naturally occurring exchangeable cations provided effective functionalization of the sorbent surface since they can induce specific sorbate-to-sorbent energy interactions. Adsorption isotherms for ion exchanged SAPO-34 sorbents show a non-linear behavior indicating that a surface functionalization was achieved to some extent.
In addition to SAPOs, we are developing novel nanoporous materials with specific framework topologies and novel compositions based on synthesis modification of titanosilicates (TS). For instance, some non-templated titanium silicates showcase a pore gating feature suitable for adsorption of molecules based on size-exclusion principles. With the addition of a molecular organic structure-directing agent (SDA) to the TS material synthesis path, the resulting framework displays a greater total pore volume and higher adsorption capacity. In addition, the new sorbent material has physical geometry characteristics and selectivity features similar to that of Chabazite and soft TS materials, respectively. A modified titanium silicate based on a Zorite starting composition was already synthesized in our labs. The preliminary data showed that a complete modification of the original Zorite framework was obtained, including the apparent formation of cages. Furthermore, different methods for SDA removal (e.g. solvent extraction, ion exchange) were studied to finally obtain a high surface area (~ 500 m2/g) sorbent with selectivity and high adsorption capacity toward gas molecules like N2.
Our results have demonstrated that both SAPO-34 and modified TS are promising nanoporous materials for large and selective adsorption of gas molecules. Future work will focus in optimizing post-synthesis modifications and/or hydrothermal synthesis.