426c Selective Reduction of Nox with H2, Co and Ch4 in Synthetic and Real Exhaust Gas of a Lean-Burn Engine
Catalytic reactions of NO/H2, NO/H2/CO, NO/CH4 and NO/H2/CO/CH4 were studied with palladium-supported zeolite MOR and zirconia-based oxide catalysts with and without O2 and H2O. The zeolite-supported catalysts reached very high NOx (NO+NO2) conversion compared to the zirconia-supported catalysts and other catalysts reported in the open literature. The catalyst operated well in the 423-623K range under NO/H2/CO/O2/H2O conditions, which is among the widest temperature window of operation reported for this condition at hand. Typical catalysts reported in the literature form N2O as a by-product and consequently exhibit low selectivity values towards nitrogen (lower than 60%) under NO/H2/O2 and NO/H2/CO/O2 reaction conditions. On the contrary, the MOR-supported catalyst showed N2 selectivity values higher than 90% in the whole 373-623K range. Pd-MOR shows synergic co-operation between H2 and CO between 423 and 473K. The formation of N2O is prevented by the presence of both H2 and CO together with oxygen in the feed, which will be the case in lean engine exhaust. The effect of the addition of rare earth metal (e.g. cerium) to Pd-MOR is significant in the case of H2 reducing agent but is less obvious with H2/CO mixture. In the presence of methane, cerium promotes the reaction rate of NOx reduction by oxidation of NO to NO2, which in turn reacts more easily with methane. The catalysts showed excellent stability in the presence of hydrogen, also in the presence of water in the synthetic gas mixture. The addition of high concentrations of CO2 did not affect catalyst performance.
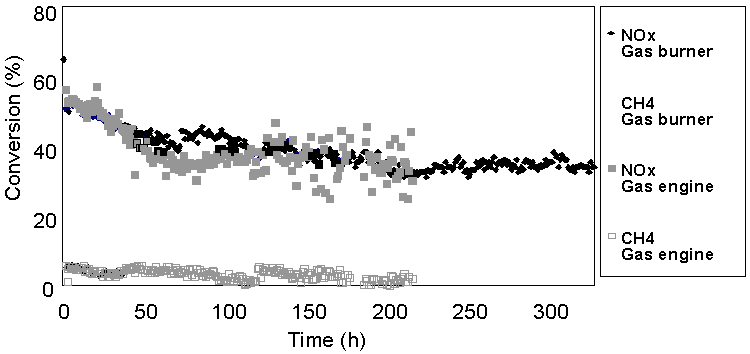
The rare-earth promoted Pd-MOR was also tested in the real exhaust gas of a reciprocating engine and burner at 673K fuelled with natural gas. The catalyst showed initial deactivation during the first days on stream. At longer time on stream, the activity persisted on a constant level (Figure 1). The initial decay of the activity, as indicated by a loss of NOx conversion by 15 %, was caused by chemisorption of sulphur species on the catalyst surface. The sulphur species originated from the odorant, which is added to the natural gas. The conversion of methane is very low: NOx is partly converted by reaction with higher hydrocarbons, CO, formaldehyde and methanol that are present in real exhaust gas. A dual bed configuration, in which an SCR and an oxidation catalyst are placed in series, can complete the conversion of CH4. Natural gas and hydrogen fuelled combustion engines could be a potential market for the zeolite-based catalysts for CH4-SCR and H2-SCR, respectively.
Figure 1