502f Hydrogen Bonds in Imidazolium-Based Ionic Liquids with –Nh2: Ab Initio and Molecular Dynamics Study
Hydrogen Bonds in Imidazolium-Based Ionic Liquids with -NH2: ab initio and Molecular Dynamics Study
Guangren Yu,†,°́Suojiang Zhang †,*
†Research Laboratory of Green Chemistry and Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100080, P. R. China
°́Graduate School of the Chinese Academy of Sciences, Beijing, 100039, P. R. China
* Corresponding author, Tel./Fax: +86-10-8262-7080, E-mail: sjzhang@home.ipe.ac.cn
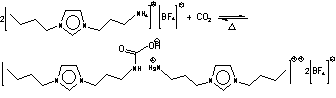 It
is one of the typical applications using ionic liquids with functional -NH2, for example 1-(3-aminoethyl)-3-methylimidazolium
hexafluorophosphate ([aemim][PF6]) and
1-(3-aminopropyl)-3-butylimidazolium tetrafluoroborate ([apbim][BF4]),
to substitute traditional aqueous
alkanolamine solutions in the fixation and
purification of CO2. Unfortunately, such ionic liquids have
undesirable high viscosity, about 2~3 order in magnitude higher than other
conventional imidazolium-based ionic liquids, which limits their eventual use
in large-scale CO2 scrubbing applications. Moreover, the mechanism
study on the CO2 fixation in such ionic liquids has not been carried
out thoroughly except a proposed "overall" reaction (J. Am. Chem. Soc. 2002, 124, 926, see to the left
scheme). In this work, the
microstructure and hydrogen bond characteristics in the above two ionic liquids
were systematically investigated by using ab
initio and molecular dynamics methods in
order to design functional ionic liquids for capturing CO2.
It
is one of the typical applications using ionic liquids with functional -NH2, for example 1-(3-aminoethyl)-3-methylimidazolium
hexafluorophosphate ([aemim][PF6]) and
1-(3-aminopropyl)-3-butylimidazolium tetrafluoroborate ([apbim][BF4]),
to substitute traditional aqueous
alkanolamine solutions in the fixation and
purification of CO2. Unfortunately, such ionic liquids have
undesirable high viscosity, about 2~3 order in magnitude higher than other
conventional imidazolium-based ionic liquids, which limits their eventual use
in large-scale CO2 scrubbing applications. Moreover, the mechanism
study on the CO2 fixation in such ionic liquids has not been carried
out thoroughly except a proposed "overall" reaction (J. Am. Chem. Soc. 2002, 124, 926, see to the left
scheme). In this work, the
microstructure and hydrogen bond characteristics in the above two ionic liquids
were systematically investigated by using ab
initio and molecular dynamics methods in
order to design functional ionic liquids for capturing CO2.
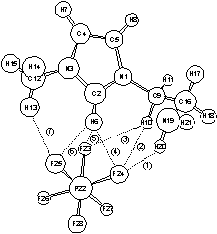
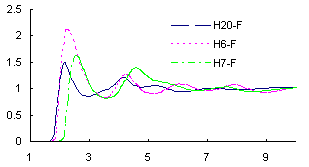
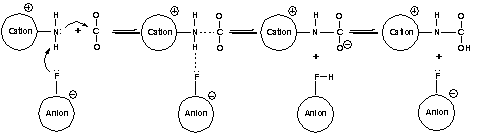
The results showed that (1) the hydrogen bonds are formed between the F-site on anions and the H-site on -NH2 of cations, along with the well-known H-site attached to C2 on imidazolium ring (see Figure 1); (2) the anion fragments potentially serve as Lewis-base catalysts in the fixing of CO2 (see Figure 2); and (3) the stronger amino-associated hydrogen bonds is one of the main factors causing high viscosity. Based on the above understandings, a type of new functional ionic liquids with lower viscosity is expected to be designed and synthesized in the near future by rationally preventing the hydrogen bonds.
Acknowledgment
This work was financially supported by National Natural Science Foundation of China (20436050).
|
|
|
|
(a) |
(b) |
Figure 1. Hydrogen bonds in the case of [aemim][PF6]: (a) ionic pair structure in the gas phase calculated at MP2/6-31G*; (b) site-site radial distribution functions between H atom on [aemim]+ and F atom on [PF6]- from a MD simulation.
|
|
Figure 2. Proposed mechanism for the role of anion in CO2 fixation.