117e A Four-Bed Psa for Clean Separation of Ethylene-Ethane Mixture
Separation of the ethylene-ethane mixture by cryogenic distillation is a well proven and mature technology. Separation of paraffins/olefins mixtures, in particular ethylene/ethane and propylene/propane, involves huge distillation columns operated at a very high reflux ratio. An US DOE study reports that around 0.12 Quads of energy is used annually for the olefin/paraffin separation by cryogenic distillation (Eldrige, 1992). We have chosen the separation of ethylene/ethane mixture for this work. Since the advent of the Skarstrom's pressure swing adsorption (PSA) for the drying of air (Skarstrom, 1960) and its extension for air fractionation (Skarstrom, 1966), PSA has become a promising alternate technology for the separation and purification of gas mixtures. There are many PSA processes presented in the open and patent literature. Here, we collectively refer to them as conventional PSA processes.
Most conventional PSA processes yield only one of the components as a pure product and at low recoveries. There are exceptions. Sircar and Zondlo, (1977) and Knaebel, (1989) have shown PSA processes that can almost cleanly fractionate air. Recently, Sivakumar and Rao, (2005) presented the fractionation of air into two clean products without a ‘waste stream'. Earlier, we classified adsorptive separations into ‘absorption-like' processes and ‘distillation-like' processes (Rao et al., 2005). Such a classification helps in understanding the mechanism of separation of various adsorption processes and improves their performance. Identifying the nature of the adsorption equilibria and the classification into distillation-like and absorption-like processes helps in the design of the adsorber. The hydrogen PSA is a good example of the absorption-like process and the oxygen-nitrogen PSA for the distillation-like PSA process. In absorption-like processes there is no need for reflux of any component, whereas in a distillation-like process both raffinate and extract refluxes have to be provided to accomplish sharp separation of a binary gas mixture. Rao, (1999) has detailed the role of reflux in separation processes. However, the requirement for refluxes seems to have not been explicitly recognized in the literature. Sivakumar and Rao, (2005) presented a mechanistic view of the absorption- and distillation-like processes.
The hypersorption process (Berg, 1951) – a moving-bed adsorption process with thermal swing much like distillation – has the potential to cleanly fractionate a given binary mixture. The Hypersorber has distinct zones as in distillation. It has a stripping and an enriching section above and below the feed point location. It has two shell and tube heat exchangers; one at the bottom for regenerating the exhausted bed by steam heating and another at the top for cooling the hot regenerated adsorbent before the adsorbent is recycled back to the stripping section. The function of the heater is similar to that of a condenser in distillation and the cooler is similar to that of a reboiler. In the cooler, the adsorbent not only cools but also gets saturated with the raffinate stream coming out of the stripping section; thus providing the raffinate reflux (adsorbate carried by the adsorbent into the stripping section). A part of extract (gas stream desorbed from the adsorbent) coming from the heater is diverted as extract reflux to the enriching section. Recently, Rao et al. (Rao et al., 2005) has shown that a sharp separation of propylene-propane mixture is possible in an ideal moving-bed adsorber with pressure swing for regeneration.
A hypersorber can be emulated in the fixed beds as depicted in Fig. 1. Each bed plays the role of the stripping, enriching, regeneration and presaturation sections during a cycle. The beds have to be switched in the direction as marked in the figure to realize a hypersorption-like operation. The stripping and the enriching zones are similar to their counterparts in distillation. The desorber and the presaturator are functionally identical to the condenser and the reboiler in distillation. The portion of the extract refluxed to the enriching bed is the enriching reflux. The raffinate amount carried in the adsorbate to the stripping bed from the presaturation bed is the raffinate reflux. An explicit identification of reflux in these terms is not available in open literature. To emulate a ‘hypersorption-like' process, we have designed a four-bed PSA setup.
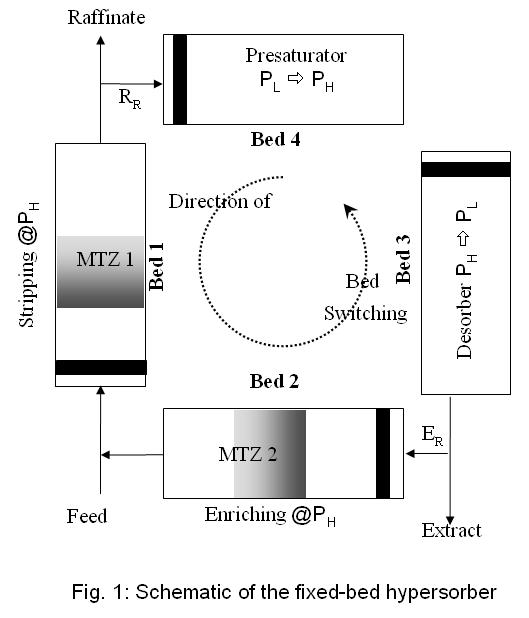
Fig. 2 shows a photograph of the experimental setup. It had four beds, each 1m long and 25 mm in diameter. They were filled with 13X-APG MOLSIVTM adsorbent supplied by UOP. The streams flowing in and out of each bed were switched synchronously at the end of each step using pneumatically activated solenoid valves. A scroll pump and a compressor were used to effect the regeneration by pressure swing. The flow of various stream were regulated using six mass flow controllers. On-line analysis of the product and intermediate streams was made using a GC, with a TCD, and a molecular sieve column. The beds were wound with heating tapes to maintain temperature above the ambient.

Fig. 2: Photograph of the experimental setup
Experiments were performed to study the effect of feed flow rate and cycle time on the performance of the PSA. The typical cycle times used were from 12-24 min. After four such switching in the direction as shown in Fig. 1, each bed would have had the role of the stripping, enriching, blowdown and presaturation sections, and a cycle was thus completed. Equal step time was used to effect pressurization, stripping, enriching and blowdown. We considered that the process attained cyclic steady-state when the product purities stabilized during which the temperature and pressure profiles also got stabilized. Cyclic-steady states were achieved for each run typically after 25 cycles. At cyclic-steady state, each bed experienced a temperature excursion from 30ºC to 80ºC and back during each cycle.
Experiments on the setup for the fractionation of ethylene-ethane mixture with 13X-APG MOLSIVTM adsorbent showed that a near equimolar mixture of ethylene and ethane can be fractionated to yield ethylene and ethane purities in excess of 95%. In a typical run – with feed rate of 1.26 SLPM (58.1 mol% ethylene), adsorption pressure of 3.18 bar, desorption pressure of 0.04 bar and cycle time of 20 minutes – the ethylene purity in the extract product stream was as high as 95.8 mol % with a recovery of 97.32 mol %. Also, the theoretical energy requirement for the evacuation and compression was 32 MJ/kmol of feed. It appears that a sharp separation of binary gas mixtures is feasible.
<>References:
1. Eldridge, R.B., ‘Olefin/Paraffin Separation Technology: A review,
2. Skarstrom, C.W., ‘Method and apparatus for fractionating gas mixtures by adsorption', US Patent 2,944,627 (1960).
3. Skarstrom, C.W., ‘Oxygen Concentration Process', US Patent 3,237,377 (1966).
4. Sircar. S., and J.W. Zondlo, ‘Fractionation of air by adsorption', US Patent 4,013,429 (1977).
5. Knaebel. K.S., ‘Pressure Swing Adsorption', US Patent 5,032,150 (1989).
6. Sivakumar, S.V., and D.P. Rao, ‘A Four-Bed PSA Unit for the fractionation of air', CHEMCON-2005, 58th Annual Session of the IIChE, 7. Rao. D.P., S.V. Sivakumar, S. Mandal, 8. Rao, D.P., ‘The futility of Raffinate Reflux Revisited', Canadian J. of Chem. 9. Sivakumar, S.V., and D.P. Rao, ‘A mechanistic view of pressure swing adsorption processes', AIChE Annual Meeting, 10. Berg. C., ‘Hypersorption Design Modern Advancements', Chem.
Web Page: www.iitk.ac.in/che/dpr.htm