504c Sulfur Resistant Metal Alloy Membranes for Hydrogen Purification
A major limitation of modern fuel cell technology is the low tolerance of fuel cell catalysts to even parts per million levels of carbon monoxide and sulfur-containing gases. For this reason, highly selective separation of the hydrogen fuel from reformate gas streams is necessary to obtain a useable feed gas; this requirement can be especially demanding for high-sulfur fuels. Perhaps the greatest challenge is that any hydrogen purification technology must itself be tolerant of reactive gases. A particularly promising method for hydrogen purification is the use of palladium and palladium-alloy thin film membranes. Although these membranes are highly efficient for exclusion of contaminant gases, sulfur compounds can block surface sites necessary for hydrogen adsorption, sometimes drastically lowering the overall permeation rate. Our research has set out to gain a fundamental understanding of how the composition of H2-permeable metal membranes can be tuned to enhance resistance to sulfides present in low-quality reformate streams.
A set of palladium-copper foils was studied. All of the foils were 60% Pd & 40% Cu. One foil was left as just the Pd/Cu mixture. The remaining foils were coated with a layer of gold. One was not heated, one was heated to 350°C and one to 480°C. A set of fresh palladium-copper foils was analyzed using atomic force microscopy (AFM) and scanning electron microscopy (SEM).
A small fresh sample of each foil was placed into a reactor, which was then filled with 1000 ppm H2S in nitrogen and pressurized to 20 psig. Samples were exposed to the sulfur for varying lengths of time ranging from 24 hours to 1 week at room temperature and also 300 ºC. The samples from each exposure were analyzed using AFM and SEM and compared to the fresh samples.
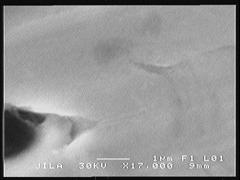
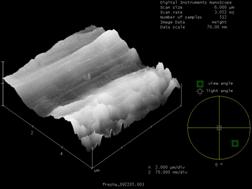
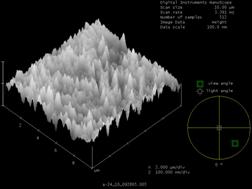
The most noticeable difference was observed on the foil without the gold coating. Prior to sulfide exposure the foil was very uniform and smooth, as can be seen in the SEM and AFM images (figure 1 and figure 2). After exposure to 1000 ppm H2S at room temperature for only 24 hours, the formation of small nodules can be seen in the SEM image (figure 3). After one week in the same conditions, the entire foil is covered with these nodules and cracking of the foil was also observed (figure 4). The nodules can also be seen in the AFM image (figure 5).
All of the gold coated samples did not exhibit this extent of nodule formation. A few widely spaced nodules were observed, but not nearly as many as the uncoated foil. This indicates that the gold layer has a large effect on the sulfur resistance of the Pd/Cu foil. The SEM image of a fresh sample of the unheated gold coated membrane can be seen in figure 6, and the SEM image after 1 week of sulfur exposure at room temperature in figure 7. The images of the other gold coated foils look very similar. Surprisingly, temperature had little effect on the foils.
We have also created an array of metal alloy membranes using dual e-beam evaporation. This technique allowed very accurate control of alloy composition and film thickness. The initial SEM and AFM analysis has been complete for all of the membranes. We are currently working on sulfur resistance and hydrogen permeation analyses.
|
|
| ||||||
| Figure 1: SEM of 60% Pd/40% Cu Foil – Fresh | Figure 2: AFM of 60% Pd/40% Cu Foil – Fresh | ||||||
|
|
| ||||||
|
|
| ||||||
| Figure 3: SEM of 60% Pd/40% Cu Foil – exposed to H2S at 25ºC for 24 hours | Figure 4: SEM of 60% Pd/40% Cu Foil – exposed to H2S at 25ºC for 1 week | ||||||
|
|
| ||||||
|
|
| ||||||
| Figure 5: AFM of 60% Pd/40% Cu Foil – exposed to H2S at 25ºC for 1 week | Figure 6: SEM of 60% Pd/40% Cu Foil Coated with Gold heated to 480°C – Fresh |