429d Influencing Chondrogenic Differentiation of Hmsc Photoencapsulated in Peg-Peptide Thiol-Methacrylate Mixed Mode Networks
Articular cartilage can be easily damaged through trauma or disease. Damaged cartilage has limited ability to induce a wound healing cascade due to lack of extensive vascularization and little cell-cell contact (Johnstone, 1998). Interest has recently emerged in developing delivery vehicles for cartilage producing cell types or chondrocytes. Yet due to the limited cell source, adult bone marrow derived human mesenchymal stem cells (hMSCs) provide an excellent alternative to using chondrocytes. In vitro, researchers have shown that hMSCs can be differentiated to form chondrocytes when treated with key environmental cues in the form of growth factors, media additives and peptide sequences.
In sharing many similar properties with tissue found in healthy cartilage, hydrogels are regarded as the class of materials most suitable for chondrogenesis. Hydrogels are highly elastic, insoluble polymer networks which when covalently-crosslinked, force the cells into a rounded morphology; similar to that of native chondrocytes, providing structural support for encapsulated cells. Poly(ethylene glycol) (PEG)-based polymers have been frequently used in biomedical applications and provide a neutral environment for cells. Although many cell types have been encapsulated within PEG-based hydrogels and have shown exceptional survivability, the viability of hMSCs is vastly decreased in these scaffolds. Unfortunately, the hydrogel scaffold provides a non-adherent environment for the cells, causing the anchorage-dependent hMSCs to undergo apoptosis. Previous research suggests that the incorporation of adhesive peptide sequences, such as RGDS (arginine-glycine-aspartic acid-serine), a well studied sequence found in fibronectin, will initiate cellular binding and enhance the viability of cells within the hydrogel (Nuttelman, 2004). Research also shows that the RGDS sequence affects chondrogenic differentiation as hMSCs upregulate fibronectin at the onset of differentiation down the chondrogenic path (Tavella, 1997). The upregulation of fibronectin allows integrins on the surface of undifferentiated hMSCs to briefly bind, providing time for cell-cell interactions, condensation and expression of chondrogenic differentiation markers, such as GAGs, before downregulation of fibronectin and further differentiation.
Thereby, modification of polymer networks with specific peptide sequences such as RGDS, will both enhance cell survivability and differentiation. Incorporation of a peptide sequence into our networks is achieved through a thiol-containing cysteine groups engineered into the peptide sequence. LeCamp et al. introduced the mechanism for photoinititated thiol-methacrylate networks (Lecamp, 2001) whereby ultraviolet light excites a photoinititator, which then abstracts a hydrogen from the thiol group of a cysteine found in the peptide of interest. This reaction proceeds in a mixed-mode fashion whereby step-wise growth (i.e., thiol-methacrylate reaction) and chain polymerization (i.e., methacrylate homopolymerization) simultaneously occur. Figure 1 displays a schematic of a crosslinked peptide network.
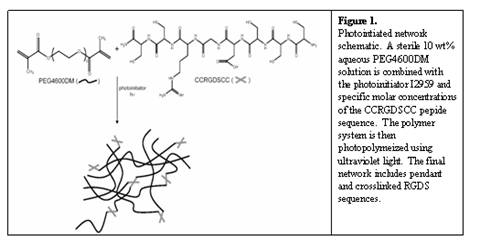
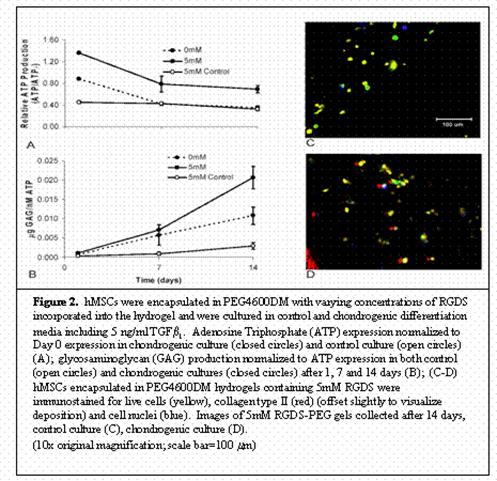
In exploring means to alleviate significant decreases in cellular viability, various concentrations of the RGDS peptide sequence were analyzed. A concentration of 5mM was found to promote the highest level of cellular survivability in these gels reaching 75% viability in control culture after two weeks. Further studies determined the extent of chondrogenesis seen in those viable cells. hMSCs encapsulated in PEG4600DM gel with 5mM RGDS and cultured in chondrogenic media supplemented with 5 ng/ml TGFb1 for two weeks showed increased viability and GAG deposition over both a 0mM chondrogenic culture and a 5mM control culture. Figure 2 displays these results. Immunostaining techniques of these samples show live cells, collagen type II deposition, and distribution of cell nuclei. As displayed in Figure 2, collagen type II, a later stage differentiation marker, is seen in the pericellular region of those live cells that have progressed down the chondrogenic pathway. Collectively, these quantitative and qualitative results establish that the incorporation of RGDS sequences in PEG gels provide the beginning stages of an extracellular matrix native to differentiating hMSCs and aid in initiating chondrogenesis.
The incorporation of an adhesive peptide sequence such as RGDS for use in chondrogenic differentiation reaches limitations. Therefore, it is imperative to create a crosslinked hydrogel network which allows for RGDS binding up until a certain point whereby it is then cleaved and released from the environment, mimicking the upregulation and downregulation of fibronectin in native cultures. As hMSCs undergo chondrogenic differentiation, the MMP-13 enzyme is upregulated around day 7, which coincides with the downregulation of fibronectin in culture (Sekiya, 2002). Our work focuses on a peptide sequence containing the thiol-reactive cysteine end group, an aggrecan-MMP-13 binding domain and the RGDS sequence. The concentration of MMP-13 produced by hMSCs in the hydrogel culture provides enough enzyme to properly cleave the RGDS sequence and release it from the network. (data not shown).
The research presented here focuses well studied polymerization techniques into a novel hydrogel system in which peptide sequences provide crosslinks. Through incorporation of RGDS attached to the cleavable sequence, we aim to provide for the natural upregulation and downregulation of this necessary peptide sequence in chondrogenic differentiation.
ADDIN EN.REFLIST Johnstone, B., T. M. Hering, et al. (1998). "In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells." Exp Cell Res 238(1): 265-72.
Lecamp, L. H., F.; Youssef, B.; Bunel, C. (2001). "Photoinitiated cross-linking of a thiol-methacrylate system." Polymer 42(7): 2727-2736.
Nuttelman, C. R., M. C. Tripodi, et al. (2004). "In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels." Journal of Biomedical Materials Research 68A: 773-782.
Sekiya, I., J. T. Vuoristo, et al. (2002). "In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis." Proc Natl Acad Sci U S A 99(7): 4397-402.
Tavella, S., G. Bellese, et al. (1997). "Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation." J Cell Sci 110 (Pt 18): 2261-70.
ADDIN EN.REFLIST