572d Liquid Phase Nitrate Reduction
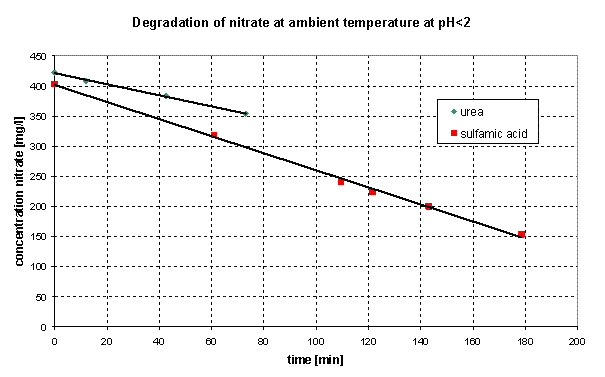
NOx elimination from dilute gaseous carriers as well as chemical reduction of nitrate laden aqueous effluents is still a major obstacle in emission control. NOx absorption, basically of very poor efficiency because of very high Henry constants [e.g. HNO=28400 bar], can be enhanced by liquid phase reaction of the dissolved constituents. Nitrogen oxides undergo several oxidation reactions in the bulk phase of aqueous solutions [1] by forming nitric acid, nitrous acid and nitrogen monoxide. These liquid phase reactions do not sufficiently contribute to absorption enhancement. Several liquid phase reduction reactions have therefore been investigated for their enhancement capability, considering the mechanisms, thermodynamics and kinetics. While chemical nitrite reduction with urea or sulfamic acid is state of the art, chemical reduction processes for nitrate reduction at ambient temperature to rapidly form molecular nitrogen [2] are still not available. Target of the present project has therefore been the investigation of liquid phase reduction reactions of acidic nitrate solutions with a focus on catalytic acceleration of nitrate reduction with urea at ambient temperature. As shown in Figure 1 it is possible to overcome energy of activation for nitrate reduction with urea and sulfamic acid at ambient temperature, enabling efficient reduction. With the reducing agents in excess reduction of nitrate to molecular nitrogen below pH 2 follows 0 order reaction.
Figure 1: Degradation of nitrate by reducing agents at ambient temperature
[1] Roiron, J.; Nitrogen 88- British Sulphurxs 12th International Conference, Geneva, 1988 [2] Fanning, J.C., Coordination Chemistry reviews, 199, 2000, 159-179