536b Development of Heterogeneous Group 4 Metallocenes: Synthesis, Application and Molecular Modelling
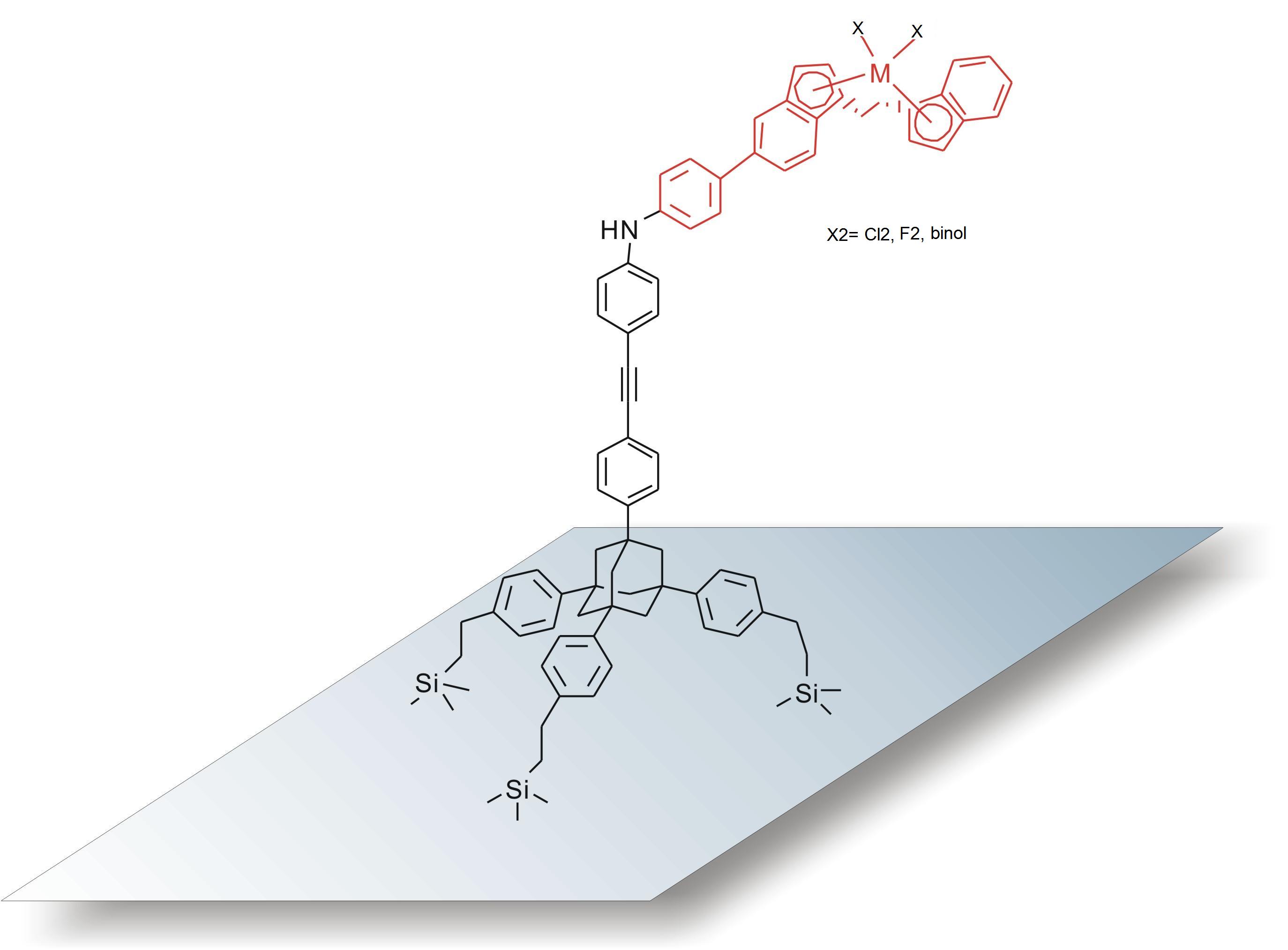
Chiral EBTHI-titanium complexes (EBTHI=ethylenebis(tetrahydroindenyl)) have proven to be highly active and selective catalysts for reactions such as enantioselective hydrogenations and hydrosilylations as well as polymerizations. Tremendous achievements have been made in the area of homogeneous chemical catalysis as well as in the implementation of these soluble complexes in industrial applications. However, such processes are not having as much impact on an industrial scale as may have been expected. The reasons may be that homogeneous catalysts lack the advantages of heterogeneous catalysts like easy separation and recycling of the catalysts, prevention of metal leaching, improvement of stability, etc. In general, heterogeneous Group 4 metallocenes are expected to show high catalytic activity and selectivity when used as hydrosilylation catalysts. Although EBTHI-titanium analogues have not been immobilized so far, significant work has been done on the immobilization of various titanocene and zirconocene polymerization catalysts, which can provide important guidelines for immobilizing EBTHI-Ti-complexes. In this project we present novel methods to prepare highly active metallocene complexes which (i) include rigid (tripodal) tethers in order to control the distance of the catalytic moiety from the surface as well as the distance between the grafted complexes (ii) show no or negligible metal leaching (iii) are highly active and selective for applications in pharmaceutical industry and (iv) can be used to develop bifunctional catalysts, i.e., solid supports that feature two or more catalytic compounds that are able to catalyze multistep reactions in a one-pot synthesis. The development of these heterogeneous catalysts involves the preparation of functional EBI (EBI= ethylenebis(indenyl)) ligands using a four step synthesis [1] in order to introduce functional tethers of various lengths and functionality to the metallocenes. Rigid (tripodal) tethers are then coupled to the homogeneous EBTHI- and EBI-titanium complexes and are used for the covalent immobilization of the catalysts. The immobilization reaction is carried out via UV-mediated hydrosilylation [2], which allows highly controlled grafting under mild conditions (no harsh chemicals, no catalyst, reaction at r.t.). State of the art surface characterization methods are used in order to investigate the distribution and concentration of active sites on the surfaces. The development and optimization of the new metallocenes is accompanied by the simulation of molecules with molecular modeling using DFT methods (DFT = Density Functional Theory) to support the synthetic attempts as well as mechanistic investigations. The performance of the new heterogeneous metallocenes will be compared to those of classic homogeneous catalysts, e.g., (R,R,R)-EBTHITibinol or (S,S)-EBTHITiCl2. After activation with different bases and silanes these catalytic systems have proven to be potent catalysts for the hydrosilylation of imines leading to chiral amines with high yields (>98%) and enantioselectivities. The investigation of the reaction kinetics has revealed a strong dependence on reaction temperature and reactant concentration. While there is a general consensus that the key intermediate in titanocene catalyzed hydrosilylations is a Ti(III)-H intermediate [3,4], we propose a catalytic cycle involving a Ti(IV) species based upon our kinetic data, analytical confirmation and mechanistic studies using DFT modelling. [1] Panarello A. P., Vassylyev O., Khinast J. G., Synlett, 2005, 5, 797 [2] Langner A., Panarello A., Rivillon S., Vassylyev O., Khinast J.G., Chabal Y.J., J. Am. Chem Soc., 2005, 127, 12798 [3] Verdagauer X., Lange U.E.W, Buchwald S.L., Angew. Chem. Int. Ed., 1998, 37, 1103 [4] Rahimian K., Harrod J.F., Inorg. Chim. Acta, 1998, 270, 330