476bf Effect of Ceria on Cu-Zn-Alumina Catalyst for Oxidative Steam Reforming of Methanol
Introduction: Hydrogen will play a major role in the future as a carbon free energy carrier. Its use in vehicles via polymer electrolyte membrane (PEM) fuel cells offers no toxic emissions and higher efficiencies compared to internal combustion engines, however efficient and safe storage of hydrogen with high energy density is still facing some technical problems. One promising solution is, on board production of hydrogen using liquid organics. Methanol offers several advantages for the hydrogen production compared to other liquid organics [1,2]. For the conversion of methanol to hydrogen different chemical reactions can be applied. The simplest option is the methanol decomposition (MD), CH3OH « CO + 2H2, DH0 = 91 kJ mol-1 (1) Yielding hydrogen and carbon monoxide. However, the high content of latter in the product gas makes this reaction unsuitable for the PEM fuel cell applications. Another reaction is partial oxidation of methanol (POM), CH3OH + ½ O2 « CO2 + 2H2, DH0 = -192.3 kJ mol-1 (2) Reaction (2) is a highly exothermic which leads the problem of heat removal and reactor temperature control and also produces significant amount of CO. Another route is the steam reforming of methanol (SRM), CH3OH + H2O « 2H2 + CO2, DH0 = 49 kJ mol-1 (3) Which is highly endothermic and have slow reaction rate. To overcome the draw backs of SRM and POM, both are combined in the present study known as oxidative steam reforming of methanol (OSRM) which can be performed auto-thermally with idealized reaction stoichiomatry. CH3OH + (1-2a)H2O + aO2 ↔ CO2 + (3-2a)H2, DH = (49.5 - 241.8*a), 0 < a < 0.5 (4) In the present investigation OSRM has been carried out over Cu-Zn-Ce-Al catalysts to improve the catalyst performance in terms of methanol conversion, hydrogen selectivity and minimization of CO formation. Experimental: All Cu-Zn-Al and Cu-Zn-Ce-Al (abbreviated as CZAi and CZCeAi respectively) catalysts were prepared by co-precipitation method using respective nitrate solutions and sodium carbonate as a precipitator. Nitrates were converted in to the oxides by calcinations in the presence of air. Pellets were made from the catalyst flakes and then crushed to a size of 20/25 mesh. Surface area and pore volume of catalysts were measured using ASAP 2010 Micromeritcs.
Table 1. Properties of catalysts CZA1 CZA2 CZCeA1 CZCeA2 CZCeA3 Cu/Zn/Al Cu/Zn/Al Cu/Zn/Ce/Al Cu/Zn/Ce/Al Cu/Zn/Ce/Al Composition, wt% 30/30/40 30/20/50 30/25/5/40 30/20/10/40 30/10/20/40 SBET, m2 g-1 92 106 96 108 101 Vpore, cm3 g-1 0.26 0.32 0.28 0.34 0.29 Methanol conversion#, % 55 67 61 96 78 H2 rate#, mmol s-1 kgcat-1 137 167 152 239 194 CO formation#, ppm 488 412 439 282 328 # T=553 K, S/M=1.4 M, O/M=0.125 M and W/F=11 kgcat mol-1 s
Catalyst performance was evaluated in a fixed bed reactor (19 mm i.d.) assembled in electrically heated furnace with PID controllers. 3 gm catalyst was loaded for each run and reduced insitu before OSRM reaction. Operating temperature and contact-time (W/F) varied from 473-573 K, 3-15 kgcat mol-1 s, oxygen to methanol (O/M) molar ratio=0.1-0.5 respectively. The steam to methanol (S/M) molar ratio=1.4 and P=1 atm was kept constant. Nitrogen was used as both carrier gas and internal standard for product analysis, which was done using Nucon 5700 Gas Choromatograph in TCD and FID mode.
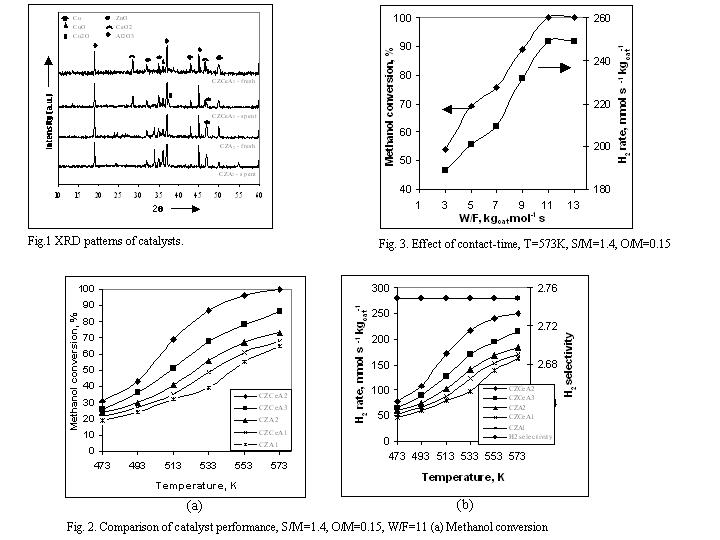
Results and Discussion: Catalyst characterization The catalyst composition, surface area and pore volume of different catalysts are shown the Table 1. XRD patterns as shown in Fig. 1 indicate that the copper was present in Cu form in fresh reduced catalyst, whereas Zn, Ce and Al in oxide form. In the spent catalysts Cu converted partly in to CuO and Cu2O due to oxidative environment of OSRM. Effect of ceria over catalysts performance From Fig. 2 (a), (b) it can be clearly seen that the addition of ceria in CZAi catalyst improved the catalysts performance greatly. The CZCeA2 exhibited 99% methanol and 249 mmol s-1 kgcat-1 hydrogen rate at 573 K whereas 65 % and 161 mmol s-1 kgcat-1 given by CZA1 . The higher conversion with CZCeA2 was due to higher surface area and better copper dispersion that was confirmed by the XRD patterns. The peak width of copper for a CZCeA2 catalyst is broader than the CZA1 catalyst as shown in Fig. 1, which resulted in a smaller copper crystallite size. Smaller the crystallite size better the copper dispersion and more copper surface area can be achieved which would enhance the catalyst activity. The selectivity, moles of H2 produced per mol of methanol converted, was almost constant, however it was found slightly lesser than the stoichiometrically obtained by reaction (4).
The effect of contact-time (W/F) on the methanol conversion and hydrogen production rate at a temperature 573 K is shown in the Fig. 3. Methanol conversion and corresponding hydrogen production rate increased with contact-time. The methanol conversion reached to almost 100% at contact-time more than 11 kgcat mol-1 s. The oxygen/methanol (O/M) molar ratio is expected to have a strong influence on the catalytic performance because the OSRM is the combination of POM and SRM reactions. The data were collected by keeping the steam to methanol molar ratio at 1.4. The methanol conversion and hydrogen production rate increased with increasing O/M ratio up to 0.15. At that ratio methanol conversion reached to a limiting value, and beyond that the rate of H2 production declined with further increase in the O/M ratio to 0.50.
Based on the product distribution different following reaction pathway has been proposed for OSRM CH3OH + 0.25O2 ↔ 2H2 + 0.5CO2 + 0.5CO (5) CH3OH + H2O ↔ 3H2 + CO2 (6) CH3OH ↔ 2H2 + CO (7) CO +H2O ↔ H2 + CO2 (8) CO + 0.5O2 ↔ CO2 (9) H2 + 0.5O2 ↔ H2O (10) CH3OH + 0.25O2 ↔ HCHO + 0.5H2O +0.5H2 (11) As mentioned previously, the actual H2 selectivity was observed slightly lower than that stoichiomatrically given by reaction (4). It shows that the some H2 produced by reactions (5) and (7). The reduction in CO formation might be due to conversion of CO to CO2 by reactions (8) and (9). The formation of formaldehyde via reaction (11) was prevented by operating the OSRM at higher contact-times [3,4].
Conclusion: The study of effect of ceria loading on the performance of Cu-Zn-Al catalysts for oxidative steam reforming of methanol suggests that the optimum 10 wt% doping of cerium improves the catalyst performance greatly in terms of methanol conversion, hydrogen selectivity and CO suppression. The almost 100% methanol conversion with CO formation as low as 290 ppm was obtained to reduce the load on partial oxidation of CO to CO2 (PROX) before feeding the hydrogen rich stream as a feed for the PEM fuel cells. The optimum operating parameters are suggested as T=553 K, contact-time 11 kgcat mol-1 s, S/M=1.4 and O/M=0.15. References: [1] V. Agarwal, S. Patel and K.K. Pant Appl. Catal. A 279(2005) 155 [2] M. Turco, G. Bagnasco, U. Costantino, F. Marmottini, T. Montanari, G. Ramis and G. Busca J. Catal. 228 (2004) 43 [3] Geissler, K., Newson, E., Vogel, F., Truong, T.B., Hottinger, P. and Wokaun, A., Phys. Chem. Chem. Phys. 3(2001) 289 [4] S. Velu, K. Suzuki, M. P. Kapoor, F. Ohashi and T. Osaki, Appl. Catal. A, 213 (2001) 47