66b Clogging by Montmorillonite in Porous Media: Hydrodynamic and Chemical Effects
Permeability reduction caused by deposition of colloidal particles in porous media, or clogging, is important in numerous aquatic systems, including water treatment (e.g., Yao et al., 1971), aquifer storage and recovery (e.g., ASCE, 2001), and surface water-ground water interaction (e.g., Packman and MacKay, 2003). Clogging is known to depend on hydrodynamic effects, through the fluid velocity, and on chemical effects, through the stability of the colloidal particles.
With regard to hydrodynamic effects, analysis of published data sets from constant-flow filtration experiments reveals that clogging decreases with increasing fluid velocity (Mays and Hunt, 2005). However, the most extensive data set in that analysis (Veerapaneni, 1996) used synthetic colloids and filter media, destabilized latex microspheres and glass beads, leaving open the question of whether the hydrodynamic effect is applicable to natural materials. With regard to chemical effects, there is considerable evidence that the valence of the dominant counter-ion has a strong impact on soil permeability (e.g., Shainberg and Letey, 1984). Using soil samples, however, prevents analysis of clogging as a function of the specific deposit because the initial fines content is unknown. To address these shortcomings, granular filtration experiments were conducted to investigate whether the hydrodynamic effect shown by Mays and Hunt (2005) also applies to clogging in natural materials, and to measure how clogging depends on counter-ion valence.
Methods
A destabilized suspension of montmorillonite was pumped through a saturated column filled with silica sand at a constant approach velocity between 0.02 and 0.5 cm/s. Effluent turbidity was measured, converted to concentration, and used to calculate the specific deposit, σ, as a function of pore volumes eluted. The head loss across the entire filter and across the top section were measured with pressure transducers. Full details are available in Mays (2005).
Montmorillonite was selected because it is a common clay mineral that is present in soils from temperate regions, sedimentary rocks and poorly drained environments (Borchardt, 1989). This colloid also allows investigation of chemical effects on clogging, since experiments could be repeated with sodium montmorillonite (Na-montmorillonite) and calcium montmorillonite (Ca-montmorillonite). This is the first quantitative comparison of clogging by these forms of montmorillonite in a granular filtration experiment.
Results
Typical results are illustrated for Na-montmorillonite and an approach velocity of u = 0.1 cm/s, referenced as experiment #12 in Mays (2005). The normalized increase in head loss, ΔH/ΔHo-1, is plotted versus the specific deposit in Figure 1, where ΔHo is the clean bed head loss. Plotting clogging data in this manner allows comparison of diverse experiments: Normalizing by the clean bed head loss controls for varying approach velocity, and using specific deposit as the independent variable controls for varying rates of deposit accumulation.
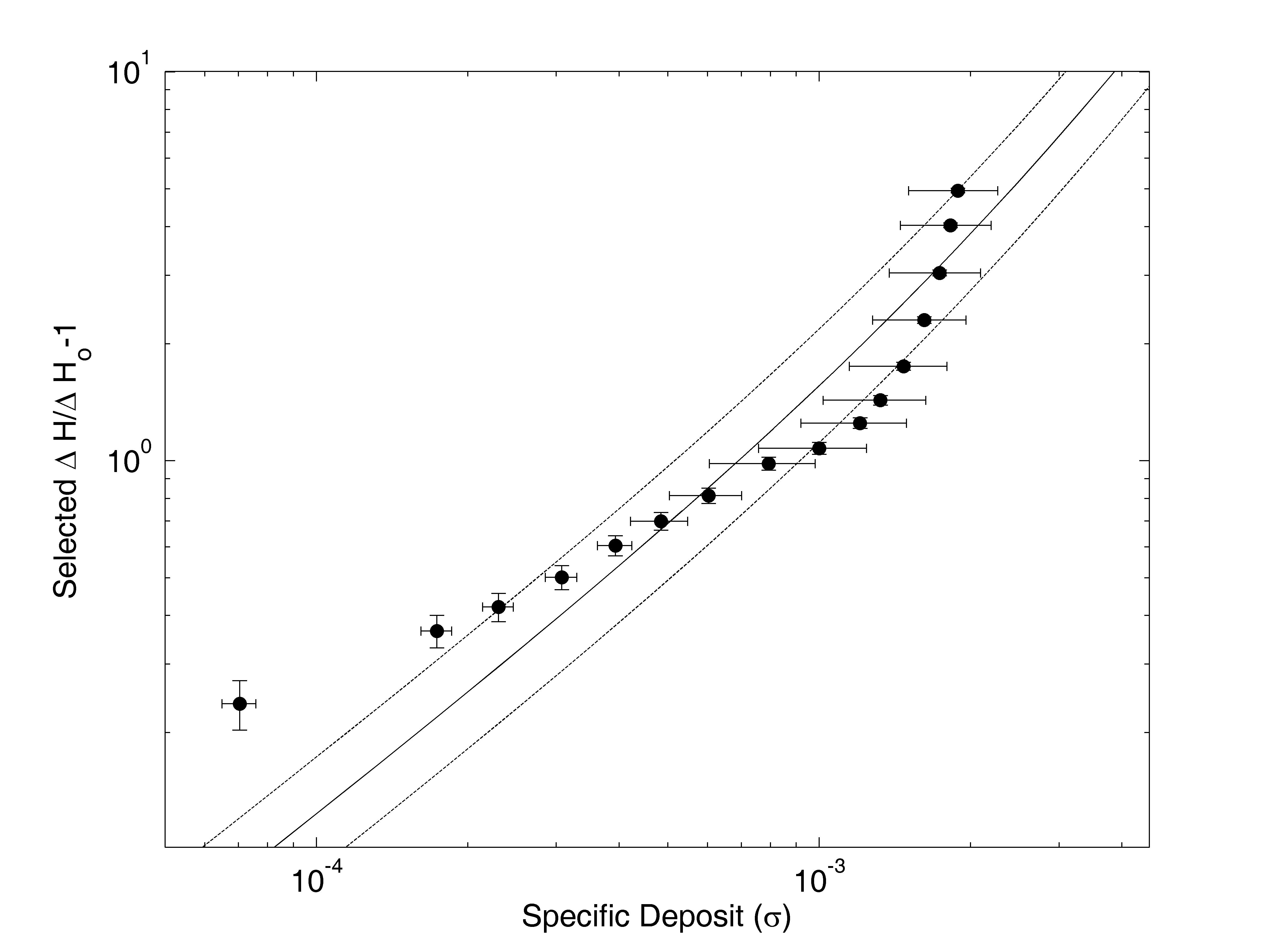
Figure 1: Clogging data for experiment #12. The fitted clogging model (γ = 600) is shown with a solid line, with plus or minus one RMSE shown by dotted lines.
A one-parameter clogging model is used to quantify the degree of clogging. This model, based on the work of O'Melia and Ali (1978), is derived in Mays and Hunt (2005):
ΔH/ΔHo = (1 + γσ)2,
where γ is a dimensionless empirical parameter that is uniquely determined by the measured data. This model can also be expressed in terms of the normalized increase in head loss:
ΔH/ΔHo-1 = 2γσ + γ2σ2,
For each experiment, the parameter γ was chosen to minimize the sum of the squared differences between the normalized increase in head loss, ln(ΔH/ΔHo-1), and the model, ln(2γσ + γ2σ2). These logarithmic expressions were used to avoid over-weighting the few largest data, and were also used to quantify the goodness of fit with the root mean squared error, RMSE. The fitted model for experiment #12 is shown on Figure 1. For this experiment, RMSE = 0.33, so on average the model differs from the data by a factor of e0.33 = 1.4, which is shown with dashed lines on Figure 1. Results for all experiments are summarized by plotting the fitted value of γ for each experiment versus Peclet number, as shown in Figure 2, which allows comparison with the analysis in Mays and Hunt (2005).
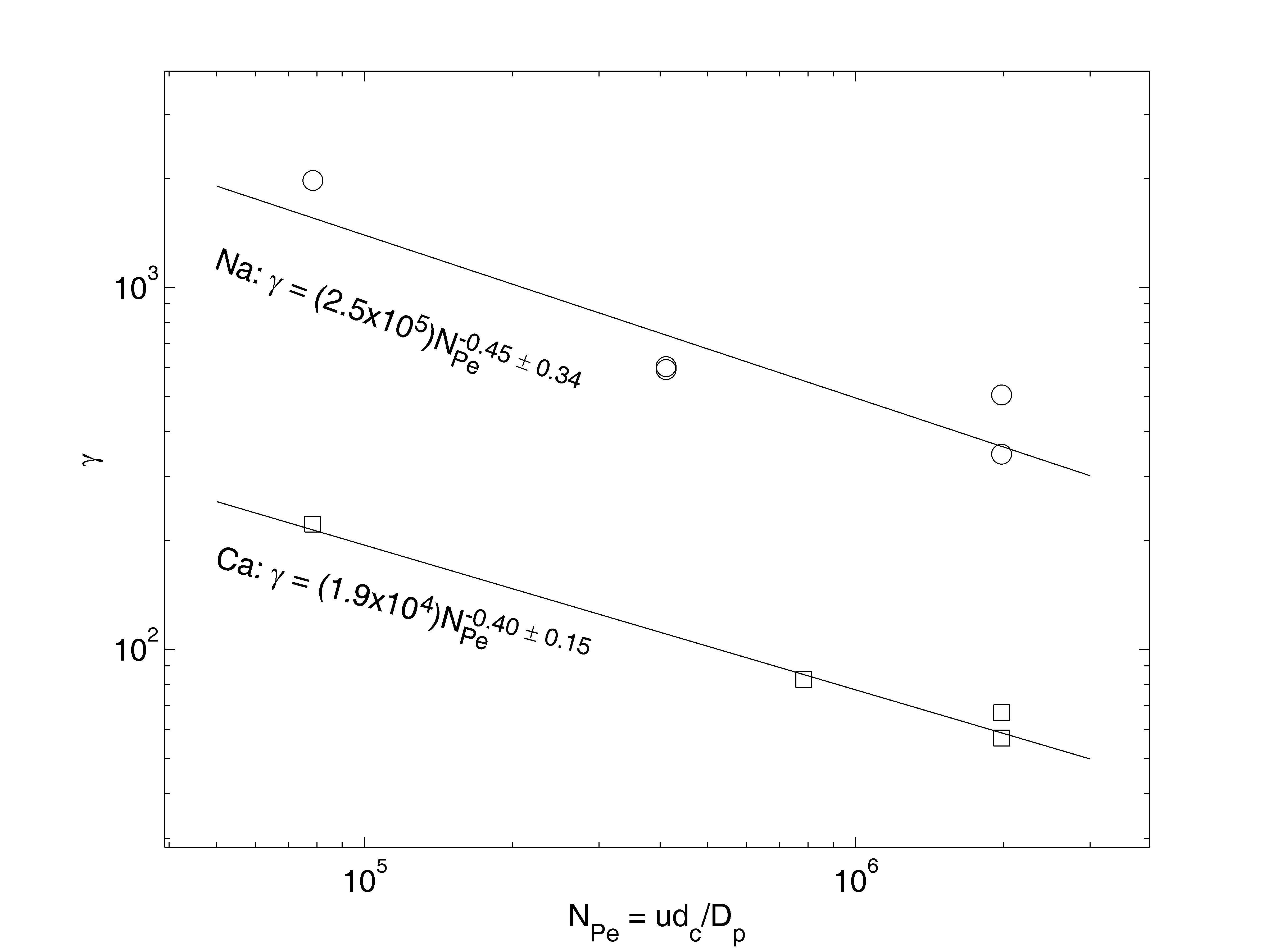
Figure 2: Fitted clogging parameter versus Peclet number for experiments conducted with Na-montmorillonite (circles) and with Ca-montmorillonite (squares), assuming primary particle diameter dp = 1 μm, fluid dynamic viscosity μ = 0.01 g/cm.s, and temperature 25oC.
Conclusions
1. The hydrodynamic effect on clogging is consistent with the eight series of experiments analyzed by Mays and Hunt (2005): For a given value of the specific deposit, more clogging is observed at lower Peclet numbers, corresponding to slower fluid velocity. The slopes of the power-law correlations shown on Figure 2 are significantly different from 0 and -1, but not significantly different from each other nor from the correlation γ ∝ NPe-0.55 reported by Mays and Hunt (2005).
2. For a given value of specific deposit, Na-montmorillonite deposits result in approximately 18 times more clogging than Ca-montmorillonite deposits (Figure 2). The ability to quantify this difference results from analyzing data from initially clean filters rather than from soil samples. This result is consistent with the known swelling behavior of Na-montmorillonite (e.g., Borchardt, 1989) that would cause increased clogging per mass of clay, and with the formation of multi-plate quasi-crystals by Ca-montmorillonite (e.g., Sposito, 1989, Chapter 10) that would cause decreased clogging per mass of clay.
References
ASCE (2001), Standard Guidelines for Artificial Recharge of Ground Water, EWRI/ASCE 34-01, American Society of Civil Engineers, Reston, VA.
Borchardt, G. (1989), Smectites, Chapter 14 in J.B. Dixon and S.B. Weed, eds., Minerals in Soil Environments, 2nd ed., Madison, WI: Soil Science Society of America.
Mays, D.C. (2005), Hydrodynamics of particle clogging in saturated granular media: Analysis and experiments, Ph.D. thesis, University of California, Berkeley, CA.
Mays, D.C. and J.R. Hunt (2005), Hydrodynamic aspects of clogging in porous media, Environ. Sci. Technol., 39(2), 577-584.
O'Melia, C.R. and W. Ali (1978), The role of retained particles in deep bed filtration, Prog. Water Res., 10(5/6), 167-182.
Packman, A.I. and J.S. MacKay (2003), Interplay of stream-surface exchange, clay particle deposition, and streambed evolution, Water Resour. Res., 39(4), 1097, DOI 10.1029/2002wr001432.
Shainberg, I. and J. Letey (1984), Response of soils to sodic and saline conditions, Hilgardia, 52(2), 1-57.
Sposito, G. (1989), The Chemistry of Soils, New York, Oxford University Press.
Veerapaneni, S. (1996), Formation and morphology of colloidal deposits in porous media, Ph.D. thesis, Rice University, Houston, TX.
Yao, K.-M., M.T. Habibian and C.R. O'Melia (1971), Water and waste water filtration: Concepts and applications, Environ. Sci. Technol., 5(11), 1105-1112.
Web Page: carbon.cudenver.edu/~dmays