690c Dehydrogenation of N-Butane over Vanadia Catalysts Supported on Theta-Alumina: Effect of Doping with K, Mg or Ce
Introduction.
Supported vanadia catalysts have been extensively studied for direct and oxidative dehydrogenation of light alkanes to the corresponding alkenes [1-3]. The catalytic properties of vanadia-based catalysts are strongly influenced by the nature of surface sites on the vanadia and also the support. As a part of a programme on selective dehydrogenation, a series of vanadia catalysts supported on alumina have been prepared, characterised and tested for n-butane dehydrogenation [4, 5]. In this paper, we present our studies on the influence of q-alumina, vanadia surface species and the dopant (K, Mg or Ce) on the catalytic properties to understand the nature of surface reaction sites responsible for the n-butane dehydrogenation and the undesired by-product reactions. Experimental
The catalyst, 3.5%V/alumina, was prepared by incipient wetness impregnation of q-alumina extrudates (trilobed, 1/20) using an aqueous oxalic acid solution of ammonium vanadate. The sample was dried at 373 K for 8 h and calcined at 823 K for 6h. Doping with K, Mg or Ce (0.5% by weight) was carried out on the above catalyst and again dried and calcined as above. Temperature programmed reduction and acidity measurements were performed using online MS. All the catalysts were tested using a fixed bed reactor. The catalyst was reduced using pure hydrogen (40 cm3min-1) for 1 hour at 873 K. The system was then purged for 30 min with Ar and n-butane was introduced (GHSV =14,400). Reaction products were analysed by GC (FID). The coked catalyst was purged with Ar at 873K for 15 minutes to remove adsorbed reactant/products and cooled to room temperature in flowing Ar. Once at 298 K, O2/Ar was introduced and MS data collected for 30 minutes at room temperature before heating the catalyst in a temperature programmed oxidation (TPO). The catalyst was then regenerated with a programmed heating rate of 10oC min-1 upto 873K and the temperature maintained until no further evolution of carbon oxides was detected. Results/Discussion
Only cracked products were found in the product stream when q-alumina was evaluated for n-butane dehydrogenation. The catalyst 3.5%V/alumina, which contains mainly polyvanadates, showed about 35% conversion with 19% butene yield. However, the by-product reaction remained unchanged after vanadia incorporation (Figure 1).
 |
Figure 1 By-product reaction on the support and catalyst
Earlier studies on chromia/alumina catalysts showed that doping the catalyst with potassium had a negative effect on the by-product reactions without affecting the dehydrogenation reaction of alkane to alkene [6]. The catalyst 3.5%V/alumina was doped with K, Mg or Ce and studied the effect of doping on the catalytic properties and deactivation mechanism. Temperature programmed reduction (TPR) of the doped catalysts (using 2% H2/N2) showed the maximum reduction peak at 843-853K whereas that for the undoped catalyst is at 800K. Indicating that the dopant is in intimately involved with the vanadia. Direct dehydrogenation of n- butane was carried out on the reduced catalysts and it was observed that undoped catalyst, which showed high activity at the beginning, deactivated within 30minutes on stream whereas the promoted catalysts retained their initial activity. The percentage conversion and selectivity to butenes with time on-stream (TOS) are shown in Figure 2.
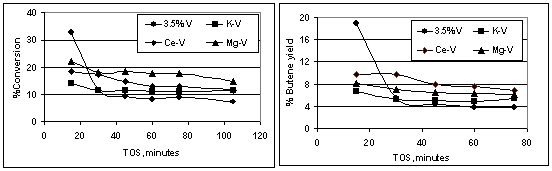 |
Figure 2 Dehydrogenation activity profiles of the catalysts
Subtle variations in the ratio of 1-butene:cis-2-buten:trans-2-butene were also observed indicating that the dopants did influence the main dehydrogenation reaction.
Coke formation on the catalyst surface is studied by temperature-programmed oxidation of the surface coke species. The total carbon formed was calculated from the TPO patterns obtained using calibrated online MS.
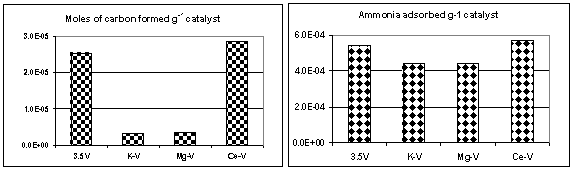
Figure 3 Carbon formation during dehydrogenation: effect of acidity.
The results showed that total carbon content increased with total acidity of the catalysts (Figure 3). However the C1 C3 yield was not affected by the presence of the dopants. Hence although the total carbon deposition is markedly affected by the presence of dopants, the cracked products are not, indicating that there is a site differential between those that laydown carbon and those involved in acid cracking. References
- Y. M. Liu, W. L. Feng, T. C. Li, H.Y. He, W. L. Dai, W. Huang, Y. C. and K.N. Fan, J. Catal., 239(2006)125-136.
- R. Grabowski and J. Słoczyński, Chem. Eng. and Proc., 44(2005)1082-1093.
- R. P. Singh, M. A. Baņares and G. Deo J. Catal., 233(2005)388-398.
- Z. Wu, H. S. Kim, P. C. Stair, S. Rugmini,. and S. D. Jackson, J. Phys. Chem. B., 109(2005)2793.
- S. D. Jackson, S. Rugmini, P. S. Stair and Z. Wu., Chem. Eng. J., in press.
- S. D. Jackson, I. M. Matheson, M. L. Naeye, P. C. Stair, V. S. Sullivan, S. R. Watson and G. Webb, Stud. Surf. Sci. Catal., 130(2000)2213.