578e Degradable Polymeric Hydrogels for Neurotrophin Elution from Implanted Prosthetic Devices
Catastrophic neural injury can result from a variety of conditions, including neurodegenerative disease, traumatic injury, or stroke. Unfortunately, neurons of the central nervous system do not spontaneously regenerate, and for most patients rehabilitation therapy and life adaptation is the best treatment option. However, in many cases upstream portions of the neural pathway remain intact and neural prostheses can be implanted to stimulate remaining nerve cells.
Neural prostheses have been successful in the auditory and central nervous system; however, they have not yet achieved the degree of control evidenced in native function. One problem in achieving this goal is the response of the tissue-device interface, which may include activation of glial cells, hemorrhage (on insertion), and encapsulation. Additionally, neuronal density near the device (i.e., within ~ 100 μm or 10 cell bodies) declines and signaling of remaining neurons may be depressed. These combined effects can increase stimulation requirements and decrease recording efficiency in implanted devices. Elimination or mitigation of these responses could dramatically increase their efficiency.
We have examined the use of neurotrophin-eluting hydrogel as a means to improve neuron connections and proximity to device surfaces. Our system consists of poly(ethylene glycol) – poly(lactic acid) (PEG-PLA) hydrogels with entrained neurotrophic factor. PLA subunits undergo hydrolytic degradation in vivo, releasing neurotrophin, and release rate can be controlled by the number of these units. Additionally, PEG has been shown to reduce protein adsorption and immune response to foreign materials, thus PEG subunits should improve biocompatibility of the tissue device-interface.
Here we report our investigations of three polymers: PEG1000LA2, PEG1540LA2 and PEG1540LA4 (PEG MW, # Lactide Units) as neurotrophin releasing elements in vitro. Release of nerve growth factor (NGF) to PC12 cells was evaluated using PEG1000LA2 and PEG1540LA4 polymers for up to 14 days. We also examined release of brain derived neurotrophic factor (BDNF) in retinal tissue slices using PEG1540LA2 and PEG1540LA4 polymers for up to 14 days. Neurite extension was evidenced in all cases, with a peak corresponding to maximum release from the polymer. Neurite length was measured quantitatively and compared to positive controls receiving a fixed NGF dose, negative controls receiving no NGF, and sham polymers, releasing bovine serum albumin.
Additionally, we placed these coatings directly on a microelectronic prosthesis, consisting of 400 μm iridium oxide electrodes on a polyimide substrate, and evaluated their adhesion and their effect on the electrochemical properties of prosthesis electrodes. We determined that charge injection capacity of the devices was slightly diminished by the presence of the coating, but electrode impedance and voltage transients remained largely unchanged. This suggests that the coatings should not impact prosthesis performance during the hydrogel degradation process.
These results demonstrate a new potential application for drug delivery polymers. These polymers can potentially improve contact at the tissue-device interface by encouraging neurite extension to the device surface, and diminishing the immune response. Ultimately, this could lead to lower excitation thresholds and improved prosthesis function.
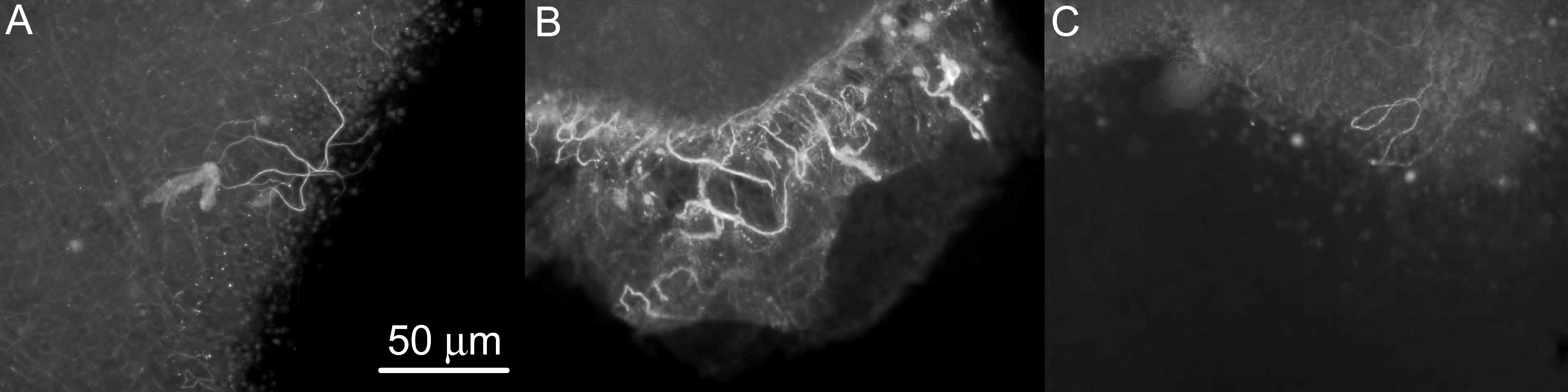
Figure 1: Neurite extension in retinal explants exposed to (A) BDNF containing PEG1540LA2 slow-releasing polymers, (B) BDNF containing PEG1540LA4 fast-releasing polymers, and (C) control receiving no BDNF. Neurites (white) were identified through staining with anti-neurofilament primary antibodies and FITC-conjugated IgG secondary antibodies.