534f Role of Mw of Negatively-Charged Polymer on Cell Colonization in 3-D Matrices
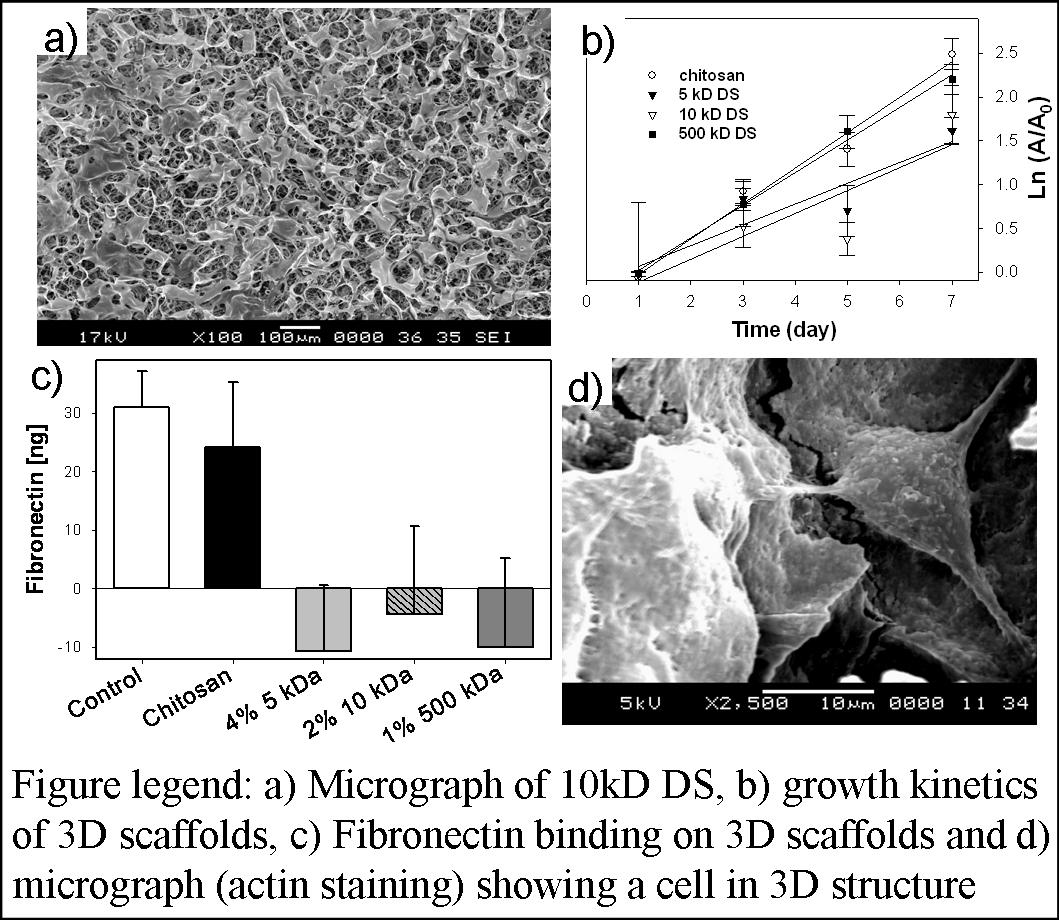
Materials and Methods. Chitosan (MW>300kD and 85% degree of deacetylation) 2D matrices were formed by air drying chitosan solution and then immobilizing DS (5kD, 10kD, and 500kD, all completely sulfated). Using 300µL of chitosan solution in each well, 3D matrices were formed inside 24-well culture plate and frozen at -20°C for at least 4 hours prior to lyophilizing overnight at -86°C. Two methods were used to form the negatively charged scaffolds: i) reacting DS with pre-formed chitosan matrix; ii) reacting DS with chitosan in solution first and then forming scaffolds similar to chitosan matrices. Various volumes, 10, 100, and 500 µL of DS were tested to determine the optimum combination in both cases. Pore structure analysis was performed using scanning electron microscopy. Amount of DS immobilized in the matrix was measured using Toluidine blue assay. To test cellular support, 25,000 and 10,000 fibroblasts were seeded on 3-D matrices and 2-D membranes and analyzed for growth by MTT-formazan assay, and spreading by actin staining and confocal microscopy. Histological analysis by H/E staining was also performed to better understand cell colonization. Further, amount of fibronectin bound to various matrices was evaluated using ELISA.
Results / Discussion: Matrices formed with 50 µL of DS solution showed optimum porosity and mechanical stability. The analysis of pore structures with the SEM showed increased surface roughness and open pore architecture in-DS-chitosan matrices. Binding DS before scaffold formation resulted in a matrix with open pores. Analysis for the quantity and stable immobilization of DS by Toluidine blue assay indicated significant presence of DS in the 3D matrices even after seven days of incubation in phosphate buffered saline solution. Immobilized DS was stable in the 3D matrices even after seven days of incubation in phosphate buffered saline solution at 37°C. Cell proliferation analysis on 3D matrices showed increased growth kinetics with increasing MW of DS. However, the trend was opposite in 2D-matrices. Cytoskeletal organization analysis showed MW of DS-dependent cell spreading (with low MW more favorable) and organized actin distribution in 3-D matrices. Analysis of fibronectin binding by ELISA showed negligible binding to all the DS-containing matrices, probably due to lack of binding domain and positive charge, unlike chitosan. Furthermore uniform distribution of cells was observed in H&E stained in the scaffolds.
Conclusions: In summary, results show significant difference in the influence of MW of DS on cell colonization. There is no influence of fibronectin affecting the cell proliferation in increasing MW of DS-containing scaffolds.