KJ3055-Chapter
8 (X-ray spectrometry)
The
x-ray tube
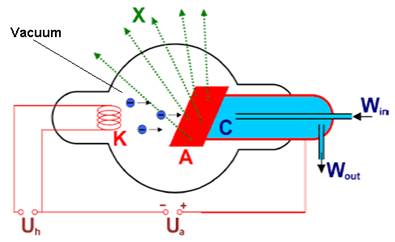
In an x-ray
tube (Fig. 1) electrons emitted by the glowing cathode K are accelerated towards the target
anode A due to the high tube voltage Ua (10 to 100 kV). As the
electron beam impacts on the target, the high kinetic energy of the electrons
is partially converted into x-ray photons. Much of electron energy is released
as heat and the target should be cooled by a water stream (W). Ua is the
heating voltage applied to the cathode.
The x-ray
spectrum produced the tube consists of bremsstrahlung
(braking radiation, continuum spectrum) and characteristic
radiation (Fig. 2). Continuum component arises from the radiation
emitted when electrons brakes when colliding with target. Characteristic
radiation is caused by electron transitions in target atoms as a result of
excitation by the electron beam. It is characteristic to the chemical element
in the target (hence its name) and appears only if the electron energy
overcomes the threshold value required to remove an inner shell electron.
Often, radiation intensity is plotted as a function of photon energy (Fig. 3).
|
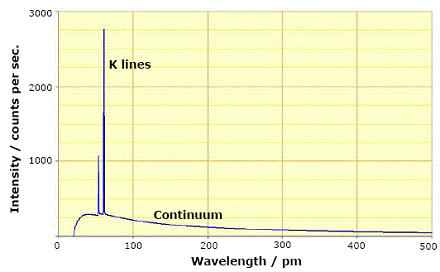
Fig. 2. Spectrum of a rhodium target tube
operated at 60 kV, showing continuous component and characteristic K lines. |
|
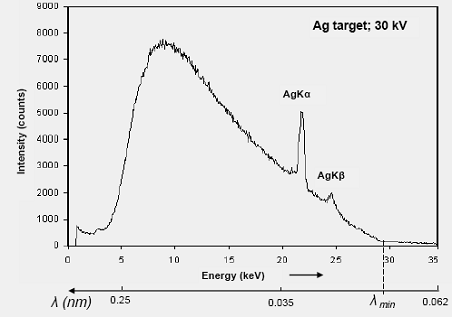
Fig. 3. X-Ray spectrum produced
by an Ag target. Notice the use of photon energy as independent variable ( |
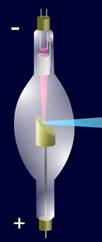
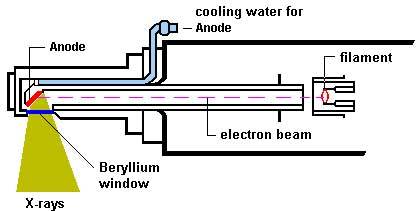
The design
of a contemporary x-ray tube is shown in Fig.
4 which displays a side-window tube. End-window tubes
are also available.
F.G. Banica,
09-03-24